Acronyms
- ADH - Antidiuretic hormone, also called arginine vasopressin
- AVP - Arginine vasopressin, also called antidiuretic hormone
- CKD - Chronic kidney disease
- CrCl - Creatinine Clearance
- DDAVP - Desmopressin, synthetic AVP
- DI - Diabetes insipidus
- ECG - Electrocardiogram
- GFR - Glomerular filtration rate
- mEq - milliequivalent
- mmol - millimole
- mOsm - milliosmole
- NKF - National Kidney Foundation
- PI - Prescribing information
- PTH - Parathyroid hormone
- RAAS - Renin-angiotensin-aldosterone system
- SIADH - Syndrome of inappropriate ADH
DEFINITIONS
- Free water - free water is water without solutes. Along the nephron, water is mostly reabsorbed with sodium and chloride. In the distal collecting duct, ADH stimulates the formation of pores (aquaporins) that allow water to be reabsorbed independent of sodium and chloride.
- Milliequivalent (mEq) - a milliequivalent is the amount of a substance it takes to combine with 1 mole of hydrogen ions. For example, 1 mole of Cl- ions will combine with 1 mole of H+ ions to form HCl. Therefore, 1 mole of Cl- ions is equal to one equivalent. If a substance is divalent, like calcium (Ca++), then only half a mole is required to combine with a mole of a monovalent ion (e.g. CaCl2). In this case, half a mole of calcium is equal to 1 equivalent. In terms of monovalent serum electrolytes (e.g. Na+, Cl-, K+), 1 mmol is equal to 1 milliequivalent, so the units of mmol/L and mEq/L can be used interchangeably. See conversions below for information on converting between milligrams, millimoles, and milliequivalents. [3,4,23]
- Mole - one mole of a substance is equal to 6.022 X 1023 units of the substance, which may be atoms, molecules, or ions. The number that defines a mole (6.022 X 1023) is equal to the number of particles in 12 grams of carbon-12. The atomic weight (or molecular weight if it contains more than one atom) of a substance is the number of grams of a substance that equals 1 mole. For example, sodium has an atomic weight of 23, so one mole of sodium would equal 23 grams. Electrolytes in serum are measured in millimoles per liter (mmol/L). If the electrolyte is monovalent (e.g. Na+, Cl-, K+), then its value in mmol/L will be the same in mEq/L and mOsm/L. See conversions below for information on converting between millimoles, milligrams, and milliequivalents. [3,4,23]
- Osmolality / Osmolarity - osmolality is a measure of the total amount of dissolved solutes in a solution. It is expressed as osmoles per kg of water. When it is expressed as osmoles per liter of water, it is called osmolarity. In regards to body fluids, the two terms are interchangeable. Osmolality differs from tonicity in that it includes all solutes dissolved in a solution, whereas tonicity excludes solutes that pass freely across membranes. Solutes that pass freely across cell membranes (e.g. urea) contribute to osmolality, but they do not contribute to tonicity. The normal range of serum osmolality is 270 - 290 mOsm/kg. Serum osmolality can be measured directly, or it can be estimated with the following formula:
- Estimated serum osmolality (mOsm/kg) = (2 X serum Na+) + (serum glucose / 18) + (BUN / 2.8)
- Where glucose and BUN are in mg/dl
- Measured serum osmolality should not exceed the estimated serum osmolality by more than 10 mOsm/kg. A difference of > 10 mOsm/kg suggests the presence of unmeasured solutes (e.g. alcohol, glycerine, mannitol). The kidneys have a remarkable ability to excrete and retain free water, and they can produce urine with an osmolality that ranges from 50 mOsm/kg (maximum dilution) to 1200 mOsm/kg (maximum concentration). [2,3,4]
- Osmole - an osmole is a measure of the number of moles of a solute that contribute to the osmotic pressure of a solution. Some molecules dissociate in solution while others do not. Molecules that dissociate form more osmotically active solutes than their parent compound. For example, sodium chloride (NaCl) dissociates in water to form Na+ and Cl-. Normal saline (0.9% NaCl solution) is commonly used as an intravenous solution because it is isotonic with plasma which has an osmolarity of 270 - 290 mOsm/L. One liter of normal saline contains 9 grams of NaCl which dissociates in solution into 154 mOsm of Na+ and 154 mOsm of Cl-. The osmolarity of normal saline is therefore equal to the sum of the independent osmoles: 154 mEq Na+ + 154 mEq Cl- = 308 mOsm/L. In actuality, it is slightly less than this because oppositely charged Na+ and Cl- do not behave completely independent of each other, so there is a correction factor that brings the effective osmolarity down to 286 mOsm/L. In contrast to NaCl, glucose does not dissociate in solution, so 1 mmol/L of glucose is equal to 1 mOsm/L of glucose. [3,4,23]
- Percent solutions - in medicine, most solutions are expressed as weight-in-volume percentages (e.g. 0.9% NaCl, 5% Dextrose). A weight-in-volume percentage is defined as grams of a constituent in 100 ml of solution. That means a 0.9% saline solution has 0.9 grams of NaCl per 100 ml or 9 grams/liter. See percent solution calculations below for information on calculating the number of millimoles and milliequivalents in a percent solution. [23]
- Tonicity - tonicity refers to the number of dissolved solutes in a solution that cannot pass freely across cell membranes. Solutes that cannot pass freely across cell membranes contribute to the osmotic gradient between intracellular and extracellular compartments, which drives the osmosis of free water. For example, urea passes freely across cell membranes, so it distributes itself equally among the intracellular and extracellular compartments, and therefore, it does not affect the osmotic gradient. In contrast, glucose does not pass freely across membranes, so increases in serum glucose raise serum tonicity. Solutes that affect tonicity are referred to as "effective osmoles," whereas solutes that do not are called "ineffective osmoles." [3,4,23]
CONVERSIONS
- Converting electrolytes from milligrams to millimoles and milliequivalents
- To convert a monovalent electrolyte like Na+ or K+ from milligrams to millimoles or milliequivalents, the electrolyte is divided by its atomic weight. The atomic weight of an ion is equal to the number of grams of the ion that make up one mole.
- Example 1
- Convert 3600 mg of sodium (Na+) to millimoles and milliequivalents
- The atomic weight of Na+ is 23 which means there are 23 grams of Na+ in a mole
- (3.6 g of Na+) / (23 g/mole) = 0.156 moles = 156 millimoles = 156 mEq
- 3600 mg of Na+ is equal to 156 millimoles of Na+. Since Na+ is a monovalent ion, millimoles are equal to milliequivalents.
- Example 2
- Convert 2300 mg of potassium (K+) to millimoles and milliequivalents
- The atomic weight of K+ is 39 which means there are 39 grams of K+ in a mole
- (2.3 g) / (39 g/mole) = 0.05897 moles = 58.97 millimoles = 58.97 mEq
- 2300 mg of K+ is equal to 58.97 millimoles of K+. Since K+ is a monovalent ion, millimoles are equal to milliequivalents.
- Converting electrolytes from millimoles to milligrams and milliequivalents
- To convert an electrolyte from millimoles to milligrams, the electrolyte is multiplied by its atomic weight. To convert millimoles to milliequivalents, multiply millimoles by the electrolyte's valence.
- Example 1
- Convert 0.86 mmol/L of magnesium (Mg++) to milligrams and milliequivalents
- The atomic weight of Mg++ is 24.3 which means there are 24.3 grams of Mg++ in a mole
- 0.86 mmol/L is equal to 0.00086 moles/L, and 0.00086 moles/L X 24.3 grams/mole = 0.021 grams/L = 21 mg/L = 2.1 mg/dl
- Since Mg++ is a divalent ion, each mole would be equal to two equivalents. Therefore, 0.86 mmol/L X 2 = 1.72 mEq/L.
- Salt calculations
- Electrolytes come in different salts for oral supplementation. For example, potassium is available as potassium chloride, potassium bicarbonate, and potassium gluconate, to name a few. The steps below show how to convert milligrams of a salt to millimoles and milliequivalents of an electrolyte.
- Example
- Calculate the milliequivalents of potassium (K+) in a 600 mg capsule of potassium chloride
- The atomic weight of K+ is 39. The atomic weight of Cl- is 35.45. The molecular weight of KCl is 74.45 (39 + 35.45).
- KCl is comprised of 52% K+ (39/74.45) so 52% of 600 mg is 312 mg of K+
- (0.312 g) / (39 g/mole) = 0.008 moles = 8 millimoles = 8 mEq of K+
- A 600 mg capsule of KCl contains 8 millimoles of K+. Since K+ is a monovalent ion, millimoles are equal to milliequivalents.
- Percent solution calculations
- Most electrolytes come in solutions that are expressed as weight-in-volume percentages of the parent salt. For example, normal saline is 0.9% sodium chloride (NaCl), and hypertonic saline is 3% sodium chloride. Weight-in-volume percentages are defined as grams of solute per 100 ml of solution, so 0.9% sodium chloride would have 0.9 grams of NaCl per 100 ml. The steps below show how to calculate the number of millimoles and milliequivalents of an electrolyte based on a percent solution.
- Example
- Calculate the milliequivalents of sodium (Na+) in 1 liter of normal saline (NaCl)
- The atomic weight of Na+ is 23. The atomic weight of Cl- is 35.45. The molecular weight of NaCl is 58.45 (23 + 35.45).
- 0.9% NaCl contains 0.9 grams of NaCl per 100 ml or 9 grams per liter
- NaCl is comprised of 39% Na+ (23/58.45) so 39% of 9 grams is 3.54 grams of Na+ per liter
- (3.54 g) / (23 g/mole) = 0.154 moles = 154 millimoles = 154 mEq of Na+ in 1 liter of normal saline
- One liter of normal saline contains 154 millimoles of Na+. Since Na+ is a monovalent ion, millimoles are equal to milliequivalents.
| Estimates of body fluid distribution | |
|---|---|
| Compartment | Estimated volume (Based on total body weight) |
| Intravascular fluid | 0.08 L/kg |
| Plasma | 0.048 L/kg |
| Extracellular fluid | 0.20 L/kg |
| Intracellular fluid | 0.40 L/kg |
| Whole body fluid (extracellular + intracellular) |
0.60 L/kg (men and children) 0.50 L/kg (women and elderly men) 0.45 L/kg (elderly women) |
| Body fat | 0.2 - 0.35 L/kg |
ELECTROLYTE DISTRIBUTION
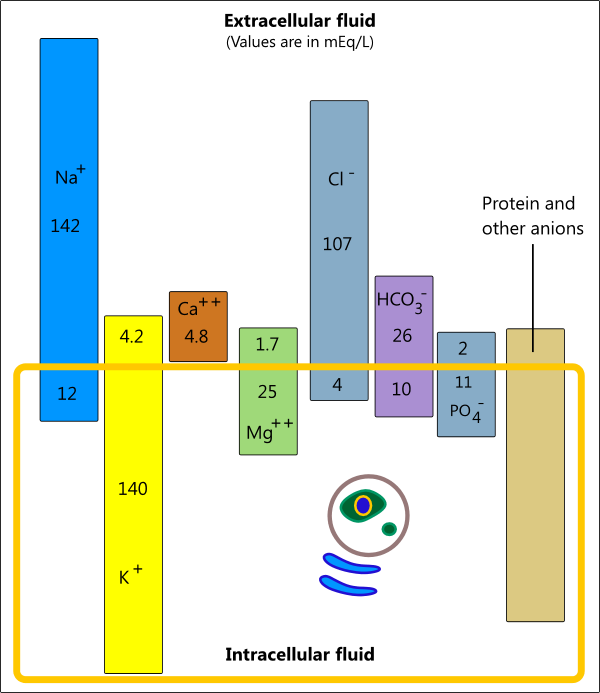
- The illustration below shows how electrolytes are distributed between intracellular and extracellular fluids. In general, most cells have a slight excess of anions giving them a net negative charge, and the interstitial fluid has slightly more cations giving it a net positive charge. [3,4]
POTASSIUM HOMEOSTASIS
- Overview
- Electrolytes are ions that have a positive (cation) or negative charge (anion). Potassium (K+) and sodium (Na+) are the two main cations in the human body. Potassium is primarily found inside cells, and sodium is the main extracellular cation. The Na+/K+-ATPase pump, which is found in the cell membrane of almost all cells, is responsible for keeping Na+ out of cells and pumping K+ into cells.
- Ions like potassium and sodium attract water molecules, so the potassium content of a cell is the primary determinant of cell volume, just as the sodium content of extracellular fluid is the main determinant of extracellular volume. The distribution of potassium across a cell membrane is important in establishing a resting membrane potential, which is involved in muscle contraction and nerve conduction. Low extracellular potassium levels cause membrane hyperpolarization and reduced excitability, which can lead to muscle weakness. Elevated extracellular potassium levels cause membrane depolarization and hyperexcitability, which can increase the risk of potentially fatal cardiac arrhythmias.
- Potassium is also involved in acid-base homeostasis and the regulation of plasma pH [3,4]
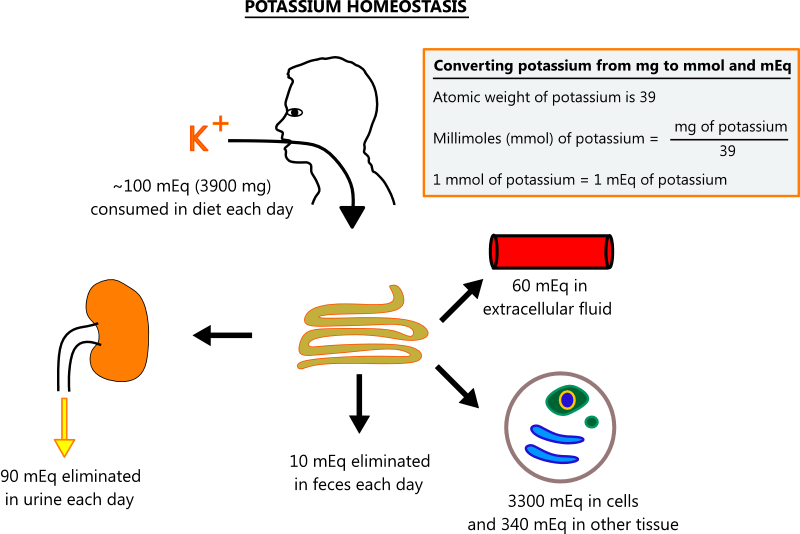
- Potassium homeostasis
- Potassium is found in most foods, and its uptake in the gut is unregulated, so most dietary potassium is absorbed. To maintain constant levels, the kidneys must excrete potassium on a regular basis. The illustration below shows normal potassium homeostasis. [3,4]
- Acute control
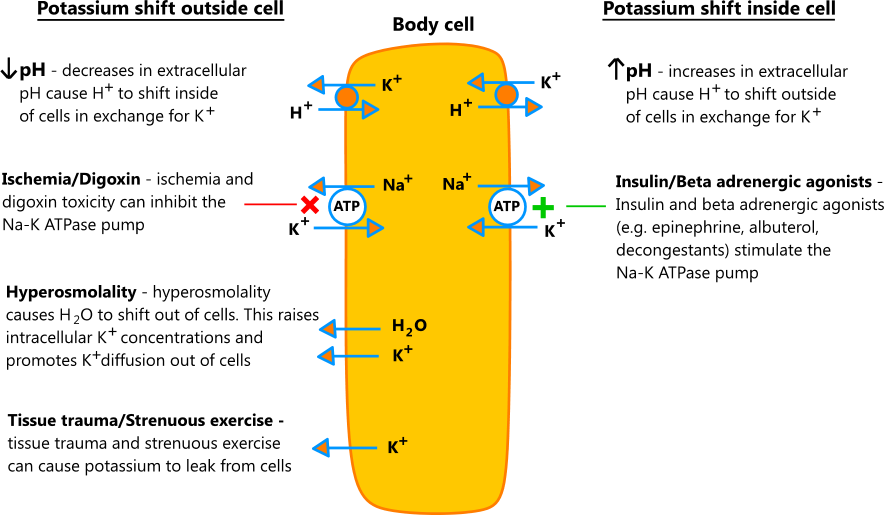
- Acutely, the body can shift potassium in and out of cells in response to changes in extracellular pH, osmolality, and certain hormones and medications. In chronic conditions, the kidneys control potassium levels, and more complex interactions are at play.
- Acute processes that shift potassium into cells do not represent true potassium deficits, and it's important to consider this when replacing potassium to avoid overcorrection. That being said, low serum potassium levels are a risk factor for arrhythmias, regardless of the etiology.
- The illustration below shows the effects of acute processes on potassium shifts [1,2,3,4]
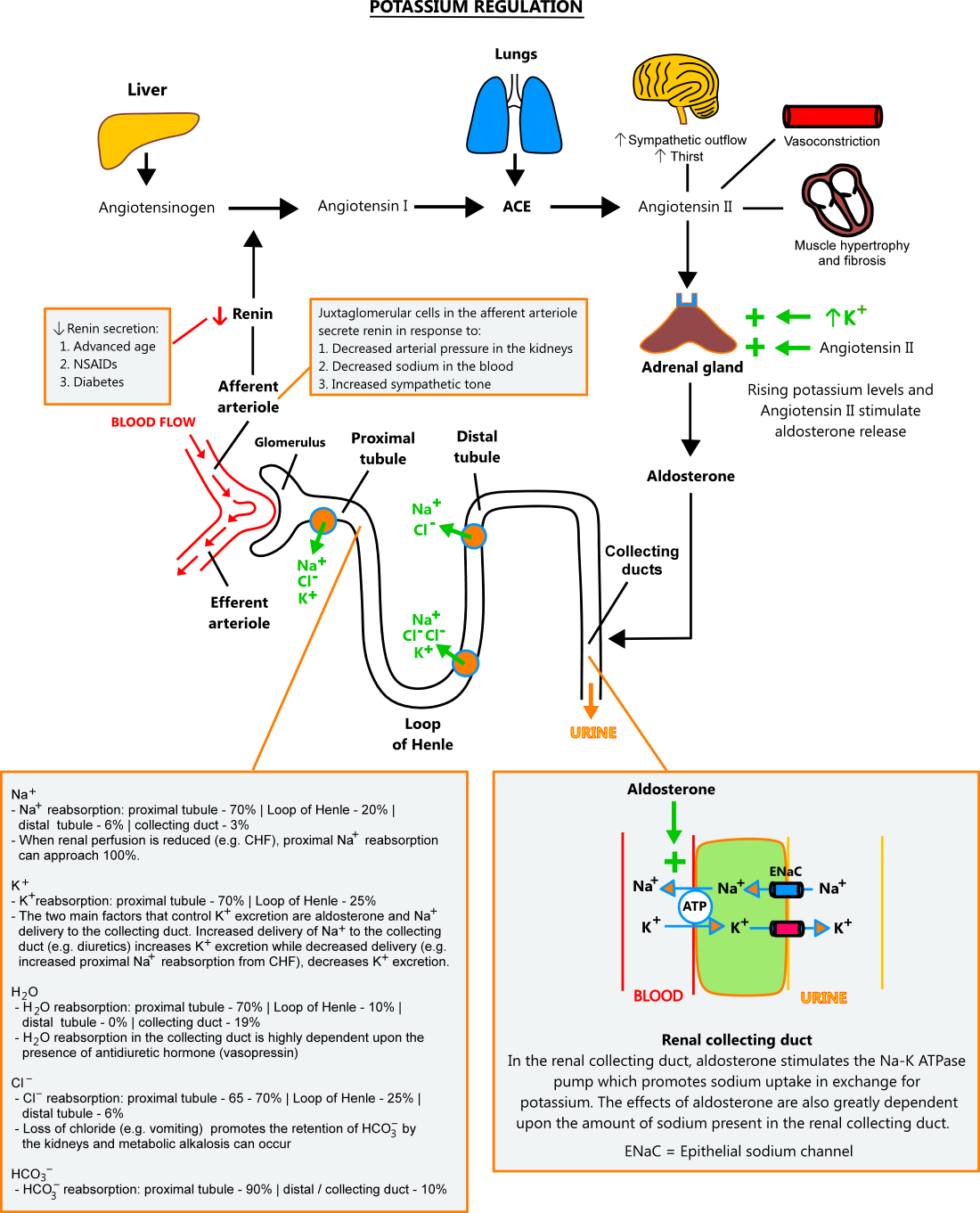
- Aldosterone / renal control
- Under normal conditions, potassium balance is primarily controlled by aldosterone and sodium concentrations in the distal nephron. Aldosterone stimulates the Na-K ATPase pump in renal collecting ducts, causing sodium to be reabsorbed from the tubule in exchange for potassium, which is then excreted in the urine. Aldosterone's effect is dependent upon the amount of sodium present in the collecting ducts; higher sodium concentrations cause greater potassium excretion and vice versa.
- Aldosterone secretion is stimulated by rising potassium levels and angiotensin II. The renin-angiotensin-aldosterone system (RAAS) produces angiotensin II in response to the following: (1) decrease in afferent arteriole pressure, (2) decreasing sodium concentrations, (3) increased sympathetic tone (see illustration below).
- Sodium concentrations in the collecting ducts depend upon its reabsorption along the nephron. Under normal conditions, 70% of sodium is reabsorbed in the proximal tubule, 20% in the Loop of Henle, and 6% in the distal tubule. When the body senses a need for sodium retention (e.g. decreased renal perfusion due to CHF, dietary sodium restriction), reabsorption in the proximal tubule can approach 100%, and almost no sodium reaches the collecting duct. In contrast, medications like loop and thiazide diuretics block sodium reabsorption along the nephron and increase its delivery to the collecting duct. [3,4,12]
HYPOKALEMIA
- Overview
- Most laboratories define hypokalemia as a potassium level < 3.5 mEq/L. Hypokalemia is probably the most common electrolyte abnormality seen in medicine, with up to 20% of hospitalized patients and 2 - 3% of outpatients having potassium levels < 3.6 mEq/L. Depending on the population, hypokalemia is seen in 10 - 40% of patients who take thiazide diuretics.
- Most patients with hypokalemia are asymptomatic and suffer no ill effects; however, patients with significant heart disease (e.g. ischemia, heart failure) are at increased risk of arrhythmias even with modest declines in potassium.
- Hypokalemia is typically secondary to an abnormal loss of potassium in the kidneys, with the most common cause being medications. Potassium loss in the stool and abrupt shifts from the extracellular to intracellular compartment are other situations that can lead to hypokalemia. Lastly, acid-base disorders have a wide range of effects on potassium that must be considered when treating these patients. See potassium regulation for more. [1,2,3,4,6,7]
| Causes of hypokalemia |
|---|
|
Potassium shift into cells - see acute control
|
|
Excessive renal loss
|
|
Other loss
|
- Symptoms / ECG changes
- Most patients with potassium levels > 2.5 mEq/L are asymptomatic. When symptoms occur, they are usually nonspecific and include weakness, fatigue, constipation, and exercise intolerance. With severe hypokalemia, muscle cramps, rhabdomyolysis, and paralysis can occur.
- ECG changes may be seen once the potassium level falls below 3.0 mEq/L, although many patients in this range will still have no findings. The absence of ECG changes should not be interpreted as low arrhythmia risk since no ECG findings or subtle changes can precede significant arrhythmias in some hypokalemic patients, particularly those with significant heart disease.
- ECG changes seen with hypokalemia include the following:
- Flattening of the T-waves - decreased T-wave amplitude is the earliest sign of hypokalemia
- ST-segment depression, T-wave inversion, PR-interval prolongation - occurs after T-wave flattening
- U-waves - U waves are positive deflections after the T-wave. In severe hypokalemia, the T-wave and U-waves fuse, forming one giant wave.
- Ventricular and atrial arrhythmias - end result in severe cases [1,6,7]
- Evaluation / Diagnosis
- In the majority of cases, the etiology of hypokalemia is obvious (e.g. diuretic use, vomiting, DKA). When the cause is not easily identified, diagnostic testing may be indicated. The table below provides a step-by-step guide for working up hypokalemia.
| Steps for evaluating hypokalemia |
|---|
Step 1 - measure urinary potassium excretion
|
|
Step 2 - based on results from Step 1, consider the following:
|
|
Step 3 - for renal potassium loss, consider the following testing:
|
- Diuretic-induced hypokalemia
- Thiazide and loop diuretics block the reabsorption of sodium along the nephron, increasing its delivery to the distal collecting ducts. High sodium concentrations in the collecting duct potentiate the effects of aldosterone while creating an electrochemical gradient that favors the excretion of potassium. Chronic diuretic therapy can also cause metabolic alkalosis, which further enhances potassium loss (see metabolic alkalosis below). [1,2,3,4]
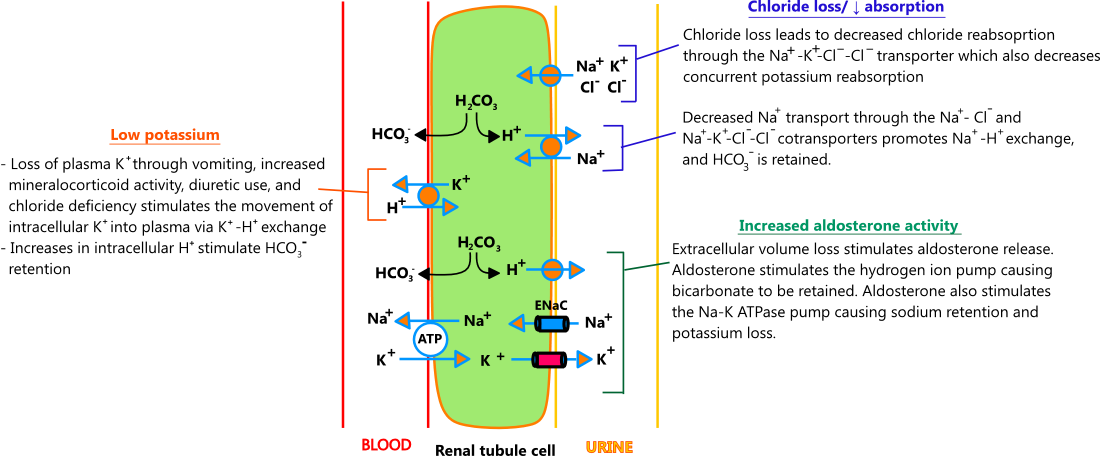
- Metabolic alkalosis
- Metabolic alkalosis is typically caused by chloride loss, which in turn, drives processes that lead to potassium excretion. Chloride loss may occur through nasogastric suctioning, vomiting, genetic defects in Na-Cl renal transporters (Bartter and Gitelman syndromes), and chronic diuretic use, the most common cause. Chronic diuretic therapy produces a mild-to-moderate metabolic alkalosis (HCO3 28 - 36 mEq/L) through chloride loss and a process referred to as "contraction alkalosis," where extracellular fluid reduction causes volume contraction around a constant amount of bicarbonate; bicarbonate concentrations rise, and metabolic alkalosis ensues.
- Acutely, metabolic alkalosis causes potassium to shift into cells in exchange for hydrogen ions. Over time, compensatory mechanisms in the kidney take over, and potassium is lost in the urine. The illustration below describes the main processes that drive potassium loss in metabolic alkalosis. [1,2,3,4]
- Metabolic acidosis
- Metabolic acidosis can lead to hypokalemia in some cases. Acutely, acidosis causes serum potassium levels to riseas as cells take up hydrogen ions in exchange for potassium. For every 0.1 decrease in arterial pH, potassium levels increase by approximately 0.6 mEq/L. As acidosis persists, renal processes predominate and hypokalemia occurs. The process behind hypokalemia in metabolic acidosis is not completely understood; proposed mechanisms for some common causes are discussed below. [1,2,3,4]
- Diabetic ketoacidosis (DKA) - DKA is thought to cause hypokalemia through the following mechanisms: (1) osmotic diuresis induced by glycosuria increases the delivery of sodium to the distal nephron where it is taken up in exchange for potassium, (2) hyperosmolar fluid in the collecting duct pulls water into the lumen causing the concentration of potassium to decrease; lower potassium concentrations in the lumen favor the diffusion of potassium from tubular cells into the lumen. Serum potassium levels often do not reflect true potassium deficits in prolonged DKA because hypertonicity, decreased pH, and insulin deficiency impede potassium uptake by cells. When fluids and insulin are given to treat DKA, profound hypokalemia is often unmasked. [1]
- Renal tubular acidosis (RTA) - renal tubular acidosis is a group of conditions where the kidneys are ineffective at eliminating acids and retaining bicarbonate. RTA type I and type II can cause profound hypokalemia. Proposed mechanisms for hypokalemia in RTA include the following: (1) increased aldosterone activity secondary to sodium loss, (2) passive diffusion of potassium into the collecting duct, (3) decreased proximal reabsorption of potassium secondary to acidemia and hypocapnia. [1,2,10]
- Carbonic anhydrase inhibitors (e.g. acetazolamide) - carbonic anhydrase inhibitors block the reabsorption of bicarbonate in the proximal tubule. This decreases the production of hydrogen ions and the subsequent reabsorption of sodium through the Na+/H+ exchanger. More sodium is delivered to the distal tubule, where aldosterone stimulates its reabsorption in exchange for potassium. See carbonic anhydrase and acetazolamide for more. [1,2,3,4]
HYPOKALEMIA TREATMENT
- Overview
- Low serum potassium levels, whether from true deficits or intracellular shifts, need to be addressed because the risk of arrhythmias is increased regardless of the etiology. That being said, correcting hypokalemia must be done cautiously because hyperkalemia has the same arrhythmogenic potential as hypokalemia.
- When treating hypokalemia, one must consider the underlying pathology because serum potassium levels do not always reflect total potassium stores. For example, in DKA, potassium levels are often normal even though potassium stores are severely depleted (see hypokalemia in DKA). Conversely, patients receiving nebulized albuterol may have low serum potassium levels when no real deficit exists. This occurs because albuterol stimulates the uptake of potassium into cells.
- The information below offers some guidance on potassium replacement, but every case must be considered individually because dietary intake, medications (e.g. ACE inhibitors, aldosterone antagonists), and medical problems (e.g. heart disease, kidney disease) must be taken into account when trying to achieve a proper balance between adequate correction and overcorrection. [1,2,3,4]
- Potassium < 2.5 mEq/L
- In general, IV potassium replacement should be given to all individuals who have a potassium level < 2.5 mEq/L
- IV potassium can be given at a rate of 10 - 20 mEq/hour. In extreme cases, 40 mEq/hour can be given through a central line under close monitoring. [6,7]
- Potassium 2.5 - 2.9 mEq/L
- Patients with significant heart disease should receive IV potassium replacement for levels < 3.0 mEq/L
- Otherwise healthy patients with normal ECG and mild to no symptoms can receive oral therapy in most cases [6]
- Potassium ≥ 3 mEq/L
- In most cases, patients with potassium levels ≥ 3 mEq/L can be treated with oral therapy
- Patients with symptoms (e.g. muscle weakness), significant cardiac disease, and/or ECG changes may need more aggressive therapy [6]
- Oral replacement recommendations
- Oral potassium supplements are the preferred method for treating hypokalemia. During chronic loss, a reduction of 0.3 mEq/L in serum potassium typically represents a 100 mEq decrease in total body potassium stores. General principles of oral replacement are discussed below.
- General principles of oral potassium replacement
- Hypokalemia treatment - hypokalemia can typically be treated with 40 - 100 mEq of potassium supplements per day. The length of therapy will depend on the deficit and may last days to weeks.
- Hypokalemia prevention - for patients with ongoing potassium losses (e.g. diuretic therapy), hypokalemia can often be prevented with 20 mEq of potassium per day
- Diuretic-induced hypokalemia - options for the treatment and/or prevention of diuretic-induced hypokalemia include the following:
- Addition of potassium-sparing diuretics (ENaC inhibitors)
- Concomitant aldosterone antagonists
- Concomitant ACE inhibitors or ARBs
- Potassium supplements
- Low-sodium diet (< 1500 mg of sodium a day)
- Dietary potassium - increasing dietary potassium (see dietary potassium below) can help improve hypokalemia, but dietary potassium is almost entirely potassium phosphate, and it will not be effective in conditions where chloride depletion coexists (e.g. diuretic therapy, vomiting). Potassium chloride supplements should be added to dietary measures in these cases.
- Hypomagnesemia - low magnesium levels impair the body's ability to retain potassium. Magnesium replacement may be necessary in some patients with hypokalemia. [1,6,7,8]
- Potassium supplements
- Oral potassium supplements come in 4 salts: chloride, bicarbonate, citrate, and gluconate. Potassium chloride is preferred in most cases because it contains chloride, which is frequently deficient in hypokalemia. Potassium citrate is mostly used to prevent kidney stones, and potassium bicarbonate is found in antacids. Potassium gluconate is present in many over-the-counter supplements.
- Potassium content can be expressed in three different ways: milligrams (mg), milliequivalents (mEq), and millimoles (mmol). One milliequivalent of potassium is equal to one millimole. When converting between milliequivalents, millimoles, and milligrams, the calculation depends on the potassium salt (see salt calculations above). The table below provides information on potassium supplements, including their conversion ratios.
| Potassium supplements | ||
|---|---|---|
| Potassium salt | Conversion (mEq of K+ per mg of salt) |
Products |
| Potassium chloride (KCL) |
1 mEq = 75 mg |
Immediate-release products
|
| Potassium citrate (K3C6H5O7*H2O) |
1 mEq ∼ 108 mg |
Tablet (Urocit-K®)
|
| Potassium bicarbonate (KHCO3) | 1 mEq = 100 mg |
|
| Potassium gluconate (KC6H11O7) | 1 mEq = 234 mg |
|
- Dietary potassium
- Potassium is found in most foods, primarily in the form of potassium phosphate. Increasing dietary potassium can help hypokalemia, but most conditions that lead to hypokalemia (e.g. diuretics) also cause chloride loss, so potassium chloride supplements are recommended.
- For healthy adults, the World Health Organization recommends potassium intake of at least 90 mEq/day (3510 mg), while the 2015 Dietary Guidelines for Americans recommends 120 mEq/day (4700 mg). To convert the milligrams of potassium in food to milliequivalents, divide milligrams by 39.
- The potassium content of some common foods is provided in the table below. Salt substitutes (e.g. No-Salt) also contain a significant amount of potassium and should be used cautiously in patients at risk for hyperkalemia. The potassium content of almost any food can be found in the USDA food database.
| Potassium content of select foods | |
|---|---|
| Food | Potassium content per 100 gram or 100 ml of select food |
| CONDIMENTS | |
Salt substitutes (e.g. No-Salt)
|
16.4 mEq per 1/4 tsp |
| FRUITS | |
| Apricots, dried | 30 mEq (1162 mg) |
| Prunes, dehydrated, uncooked | 27 mEq (1058 mg) |
| Raisins, seedless | 19 mEq (749 mg) |
| Plums, dried, uncooked | 19 mEq (732 mg) |
| Dates, medjool | 18 mEq (696 mg) |
| Avocados, raw | 13 mEq (507 mg) |
| Bananas, raw | 9 mEq (358 mg) |
| Orange juice | 5 mEq (208 mg) |
| Grapefruit juice | 4 mEq (142 mg) |
| VEGETABLES | |
| Yam, cooked, boiled, drained, or baked | 17 mEq (670 mg) |
| Spinach, fresh | 14 mEq (558 mg) |
| Parsley, fresh | 14 mEq (554 mg) |
| Potatoes, white, flesh and skin, baked | 14 mEq (544 mg) |
| Mushrooms, Chanterelle, raw | 13 mEq (506 mg) |
| Beets, cooked, boiled | 8 mEq (305 mg) |
| Carrots, raw | 8 mEq (320 mg) |
| Mushrooms, Shiitake, raw | 8 mEq (304 mg) |
| Tomatoes, raw, red, ripe | 6 mEq (237 mg) |
| Radishes, raw | 6 mEq (233 mg) |
| NUTS AND SEEDS | |
| Pistachio nuts, dry roasted | 26 mEq (1007 mg) |
| Sunflower seed kernels, dry roasted | 22 mEq (850 mg) |
| Pumpkin and squash seed kernels, roasted | 20 mEq (788 mg) |
| Almonds, dry roasted | 18 mEq (713 mg) |
| Brazil nuts, dried | 17 mEq (659 mg) |
| Cashews, dry roasted | 14 mEq (565 mg) |
| Walnuts, English | 11 mEq (441 mg) |
| Pecans | 11 mEq (410 mg) |
| BEANS | |
| Soybeans, mature, boiled | 13 mEq (515 mg) |
| Lima beans, mature, boiled | 13 mEq (508 mg) |
| Black beans, mature, boiled | 9 mEq (355 mg) |
| Chickpeas (garbanzo beans), mature seeds, boiled | 7 mEq (291 mg) |
| Peas, green, raw | 6 mEq (244 mg) |
| Beans, snap, green, raw | 5 mEq (211 mg) |
| DAIRY PRODUCTS | |
| Yogurt, low fat, plain | 6 mEq (234 mg) |
| Milk, 2% | 5 mEq (182 mg) |
| Egg, whole, cooked, omelet | 3 mEq (117 mg) |
| Cheese, cheddar | 2 mEq (76 mg) |
| BEEF | |
| Beef, round cut, pan fried | 12 mEq (484 mg) |
| Beef, tenderloin steak, grilled | 11 mEq (443 mg) |
| Beef, ground, 90% lean meat, pan-browned | 11 mEq (433 mg) |
| Beef, top sirloin steak, broiled | 10 mEq (380 mg) |
| Beef, rib, roasted | 8 mEq (319 mg) |
| Beef, brisket, braised | 7 mEq (275 mg) |
| POULTRY | |
| Chicken, breast, grilled | 11 mEq (420 mg) |
| Turkey, breast, roasted | 6 mEq (249 mg) |
| FISH | |
| Mahimahi, cooked, dry heat | 14 mEq (533 mg) |
| Trout, cooked, dry heat | 12 mEq (463 mg) |
| Salmon, coho, wild, cooked, moist heat | 12 mEq (455 mg) |
| Tuna, white, canned in water | 6 mEq (237 mg) |
HYPERKALEMIA
- Overview
- In adults, hyperkalemia is defined as a potassium level > 5.2 mEq/L. It is seen in up to 10% of hospitalized patients and 1% of outpatients. A number of conditions can lead to hyperkalemia, but the most common cause is the use of drugs that inhibit the RAAS; up to 10% of outpatients who are prescribed ACE inhibitors experience hyperkalemia within a year. Like hypokalemia, hyperkalemia can lead to cardiac arrhythmias and death if untreated.
- The kidneys are the primary regulators of potassium balance, and three main processes can reduce their ability to excrete potassium: (1) decreased delivery of sodium to the distal nephron, (2) decreased aldosterone activity, (3) diseases that damage the distal collecting duct and impair its ability to secrete potassium. Factors that control aldosterone secretion and delivery of sodium to the distal nephron are discussed above (see aldosterone/renal control). Diseases that impair potassium secretion in the distal collecting duct include diabetic nephropathy, lupus, sickle cell disease, lower urinary tract obstruction, and renal tubular acidosis, to name a few. [7,12,13]
| Causes of hyperkalemia |
|---|
|
Potassium shift out of cells - see acute control
|
|
Decreased renal excretion
|
|
Other
|
- Symptoms / ECG changes
- Most patients with hyperkalemia are asymptomatic. When levels rise above 6.5 mEq/L, some patients may experience nonspecific symptoms such as weakness, fatigue, palpitations, and paresthesias. Severe hyperkalemia (≥ 7 mEq/L) can cause respiratory paralysis and cardiac arrest.
- ECG changes in hyperkalemia are related to the degree of potassium elevation (see below). Bradycardia may be the only new finding in patients with heart disease and abnormal baseline ECGs.
- Potassium 5.5 - 6.5 mEq/L
- Peaked T-waves
- Shortened QT-interval
- ST-segment depression
- Potassium 6.5 - 8 mEq/L
- Peaked T-waves
- Prolonged PR interval
- Diminished or absent P waves
- QRS widening
- Amplified R wave
- Potassium > 8 mEq/L
- Absent P waves
- Progressive QRS widening
- Bundle branch blocks
- Sine wave pattern - fusion of widened QRS with T-wave
- Ventricular fibrillation or asystole [13]
- Evaluation / Diagnosis
- Most cases of hyperkalemia have an obvious etiology (e.g. medications, kidney failure). When the cause is not apparent, the labs below can help to identify the source. In cases where hyperkalemia is unexpected, potassium levels should be rechecked because spurious elevations are common (see pseudohyperkalemia above).
| Laboratories for evaluating hyperkalemia |
|---|
Serum creatinine and GFR / CrCl
Urine potassium
Urine sodium
Other labs that may be helpful depending on the presentation / risk factors
|
HYPERKALEMIA TREATMENT
- Overview
- Severe hyperkalemia can lead to life-threatening arrhythmias, so it should be addressed promptly. Chronic conditions like renal failure require ongoing treatment, while severe, acute elevations require immediate attention.
- Potassium levels
- There are no consensus guidelines that define an emergent potassium level, nor is there a standardized approach to treatment. The ranges below offer some guidance on treating hyperkalemia, but it's important to keep in mind that every case must be considered individually because the rate of rise, ECG changes, and etiology all play a role in determining how aggressive therapy should be.
- Potassium 5 - 5.9 mEq/L - elevations in this range are considered mild and typically can be treated with medication adjustments
- Potassium 6 - 6.4 mEq/L - elevations in this range are considered moderate, and in most cases, hospital admission is recommended
- Potassium 6.5 - 6.9 mEq/L - elevations in this range are considered moderate-high. Hospital admission with ECG monitoring is recommended, along with acute treatment.
- Potassium ≥ 7.0 mEq/L - elevations in this range are considered severe. Prompt acute treatment is recommended, along with hospital admission. [13,14,15]
- Acute treatment
- When potassium levels exceed 6.5 mEq/L, the risk of arrhythmia is increased, and acute treatment is indicated. Insulin and albuterol act quickly to shift potassium into cells, and calcium helps to stabilize cardiac transmembrane potential. Nuances of each treatment are discussed below.
- Calcium gluconate 10%
- Dosing: 1 ampule (1 gm) by slow IV bolus (1 - 2 min). May be repeated after 5 minutes if ECG does not improve.
- Onset of action: < 3 minutes
- Duration of effect: 20 - 50 minutes
- Other: if digitalis toxicity is suspected, use caution or avoid
- Regular insulin
- Dosing: 0.1 unit/kg (max 10 units) by IV bolus. If serum glucose is < 250 mg/dl, give 50 - 100 ml bolus of 50% dextrose prior to insulin. Another regimen is 20 units by IV infusion over 1 hour together with dextrose 60 g.
- Decrease in potassium: 0.6 - 1.2 mEq/L after 1 hour
- Onset of action: 15 minutes
- Duration of effect: 4 hours
- Other: monitor serum glucose hourly for at least 3 hours
- Albuterol
- Dosing: 10 - 20 mg via nebulizer
- Decrease in potassium: 0.5 - 1 mEq/L
- Onset of action: within 30 minutes
- Duration of effect: maximum effect seen at 90 minutes
- Other: more effective when used with insulin. Dose is much larger than what is used in bronchospasm and may precipitate arrhythmias in patients with heart disease. [13,15]
- RAAS inhibitor-induced hyperkalemia
- Hyperkalemia is seen in up to 10% of people prescribed RAAS inhibitors. Most patients with normal renal function tolerate RAAS inhibitors fine, while patients with diabetes, CHF, and chronic kidney disease are at risk for hyperkalemia. Despite this, RAAS inhibitors have consistently been shown to improve outcomes in all of these conditions. Providers are therefore left with the task of balancing the benefits of RAAS inhibitor therapy with the risks of hyperkalemia. The recommendations presented below provide some guidance in treating RAAS inhibitor-induced hyperkalemia.
- Recommendations for treating RAAS inhibitor-induced hyperkalemia:
- Inquire about and discontinue the following if applicable: high-potassium foods, salt substitutes, herbs and supplements that are high in potassium, NSAIDs
- If possible, discontinue medications that can raise potassium levels (e.g. ENaC inhibitors, tacrolimus, cyclosporine, ketoconazole)
- Prescribe a thiazide or loop diuretic (loop diuretics are preferred if GFR is < 30 ml/min)
- In patients with chronic kidney disease and metabolic acidosis (HCO3 < 22 mEq/L), prescribe one to two 650 mg tablet(s) of sodium bicarbonate twice a day. Each tablet contains 8 mEq of sodium bicarbonate. Alternatively, 1/2 – 1 teaspoon of baking soda daily provides 25 – 50 mEq of sodium bicarbonate.
- If potassium is < 5.5 mEq/L, decrease the dose of the RAAS inhibitor. If patient is taking multiple RAAS inhibitors, discontinue one. Recheck potassium in 1 week.
- When prescribing spironolactone, the dose should not exceed 25 mg when given with an ACE inhibitor or ARB. Do not combine spironolactone with an ACE inhibitor or ARB if GFR is < 30 ml/min.
- If potassium is > 5.5 mEq/L despite the above steps, discontinue the RAAS inhibitor [12]
- Chronic kidney disease (CKD)
- Chronic kidney disease is seen in up to 15% of U.S. adults and is a significant risk factor for hyperkalemia. Kidney disease in itself does not typically cause hyperkalemia until the GFR falls below 30 ml/min. However, many CKD patients are treated with RAAS inhibitors and have coexisting conditions (e.g. CHF) that compound the risk and cause elevations at GFRs above 30 ml/min. The recommendations below offer some guidance in preventing and treating hyperkalemia in chronic kidney disease.
- Recommendations for treating and preventing hyperkalemia in chronic kidney disease:
- Target serum potassium levels in CKD should be 4 - 4.5 mEq/L. Levels ≥ 5 mEq/L should be followed closely, and strategies to prevent further rise should be implemented.
- Dietary potassium restriction (2 - 3 grams/day or 51 - 77 mEq/day) should be started when potassium levels are > 5 mEq/L
- Sodium bicarbonate (3 - 5 grams/day) is indicated when metabolic acidosis is present (HCO3 < 22 mEq/L). A study published in 2023 found that sodium bicarbonate therapy in kidney transplant patients with metabolic acidosis did not slow GFR decline over 2 years. [PMID 36708734]
- Loop diuretic should be added or their dose increased when appropriate
- Prescribe potassium binders (see below) when other measures fail [14,15]
- Potassium binders
- Sodium polystyrene sulfonate (Kayexalate®) - sodium polystyrene sulfonate (SPS) is a cation exchange resin that binds potassium in the distal large intestine. SPS can be given orally or as a rectal enema. SPS swells when it comes in contact with water, and in large doses, it can increase the risk of intestinal obstruction. To help prevent this and speed delivery to the distal colon, SPS is commonly given with sorbitol, an osmotic cathartic. The effects of SPS are not seen for 2 hours, so it is not useful for the acute treatment of hyperkalemia. Daily doses of 15 - 30 grams are sometimes used to control mild-to-moderate hyperkalemia (5 - 5.9 mEq/L) in CKD. A small trial (N=33) in patients with CKD (average GFR 18 ml/min) and hyperkalemia (average potassium 5.24 mEq/L) found that SPS 30 grams once daily for 7 days lowered serum potassium levels by 1.04 mEq/L compared to placebo. [PMID 26576619]. SPS should be avoided in patients at risk for bowel obstruction or perforation. [SPS PI] [13,14,16]
- Patiromer (Veltassa®) - patiromer is a non-absorbed cation exchange polymer that binds potassium in the intestines and increases its excretion. Patiromer comes as a powder for suspension, and the starting dose is 8.4 grams once daily. It can be titrated at a minimum of weekly intervals to a maximum of 25.2 grams once daily. Unlike Kayexalate, patiromer does not swell appreciably in water, so it carries a lower risk of intestinal obstruction than SPS. Magnesium loss leading to hypomagnesemia is seen in up to 8% of patients. In a study of patients with CKD treated with RAAS inhibitors (average baseline potassium of 5.6 mEq/L), patiromer decreased potassium levels by 1.01 mEq/L after 4 weeks. [PMID 25415805]. In another study, patiromer was shown to be safe and effective for up to 52 weeks. [PMID 26172895] [Veltassa PI] [13,14,16]
- Sodium zirconium cyclosilicate (Lokelma®) - Lokelma is a non-absorbed zirconium silicate that binds potassium in the intestine and increases its excretion. Lokelma comes as a powder for suspension, and the starting dose for hyperkalemia is 10 grams three times a day for up to 48 hours. After that, the recommended maintenance dose is 10 grams once daily, with a maximum of 15 grams per day. Lokelma contains a significant amount of sodium (400 mg per 5 grams), and in trials, more patients on Lokelma experienced edema (5.9% with 10 g/day and 16.1% with 15 g/day) compared to placebo (2.4%). In a study of patients with an average baseline potassium of 5.6 mEq/L, Lokelma lowered serum potassium levels to 4.5 mEq/L after 48 hours and was shown to be effective at maintaining normal potassium levels for up to 28 days. [PMID 25402495] [Lokelma PI] [13,14,16]
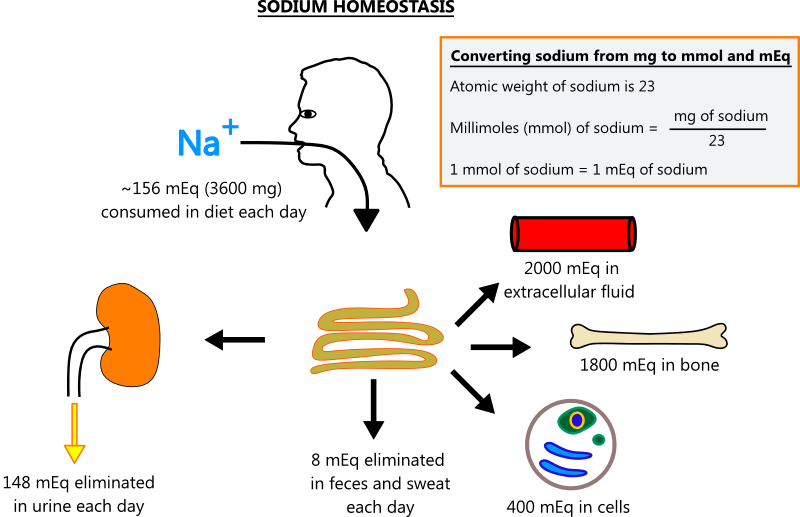
SODIUM HOMEOSTASIS
- Overview
- Sodium is the most abundant extracellular cation, and it is the primary determinant of serum osmolality, which makes it the most important solute in regulating fluid balance. Sodium homeostasis is depicted in the illustration below.
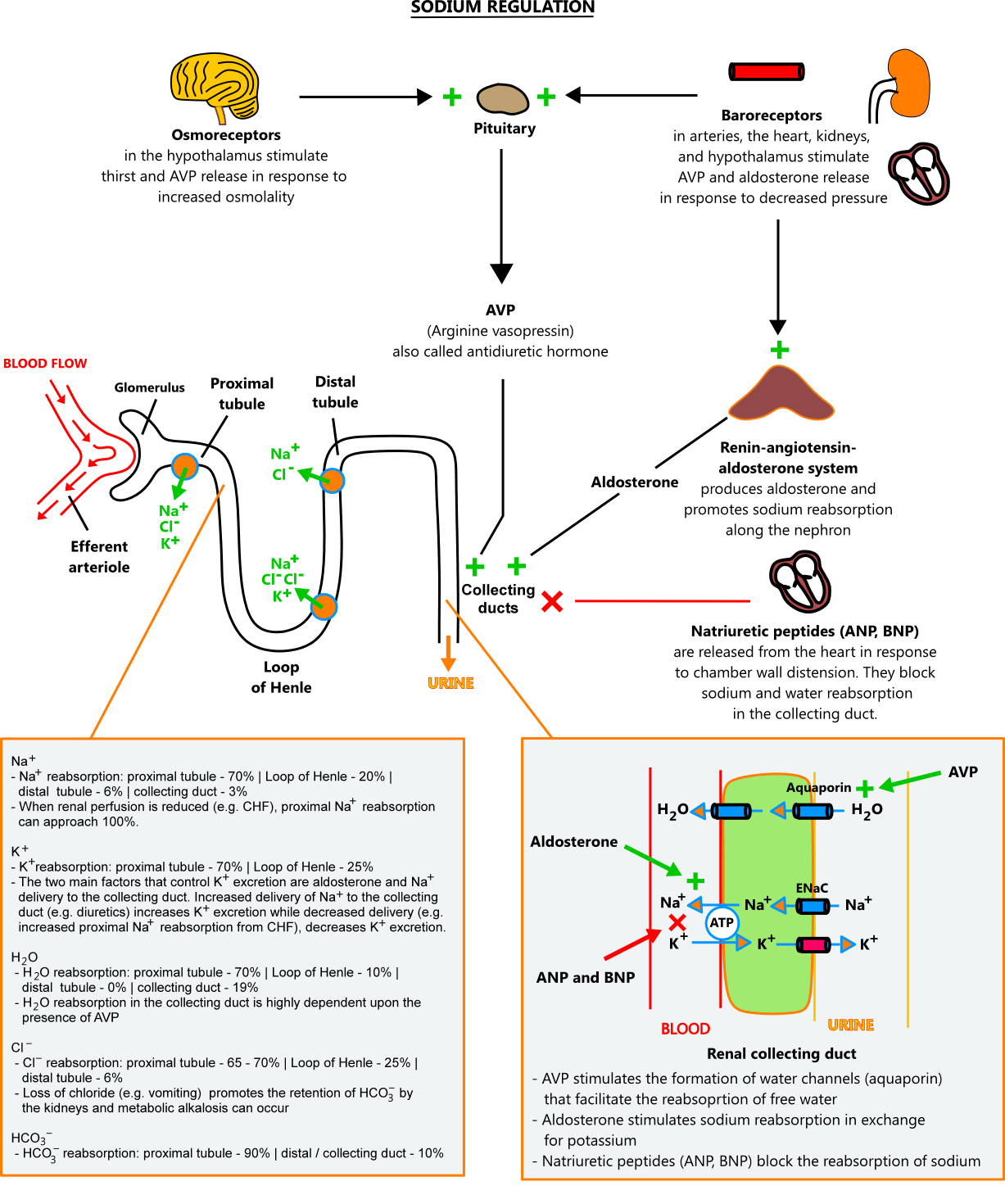
- Sodium regulation
- Given its importance in maintaining body fluid status, it's no surprise that sodium is controlled by a number of processes. The most important ones are described below.
- Arginine vasopressin (AVP) - arginine vasopressin, also called antidiuretic hormone (ADH), is a hormone released by the posterior pituitary gland in response to stimuli from osmoreceptors and baroreceptors. The main action of AVP is in the renal collecting duct, where it stimulates the reabsorption of free water (water free of solutes).
- Serum osmolality - serum osmolality is primarily a product of sodium and its accompanying anions, chloride and bicarbonate. Osmoreceptors in the hypothalamus sense serum osmolality and adjust water retention in response. When serum osmolality is high (≥ 295 mOsm/kg), they promote water conservation by stimulating AVP release and thirst. When osmolality is low (< 275 mOsm/kg), they inhibit AVP release. Serum osmolality can be measured directly or estimated with the formula below. Measured serum osmolality should not exceed the estimated serum osmolality by more than 10 mOsm/kg. A difference of > 10 mOsm/kg suggests the presence of unmeasured solutes (e.g. alcohol, glycerine, mannitol).
- Estimated serum osmolality (mOsm/kg) = (2 X serum Na+) + (serum glucose / 18) + (BUN / 2.8)
- Where sodium is in mEq and glucose and BUN are in mg/dl
- Baroreceptors - baroreceptors located in the carotid sinus, aortic arch, cardiac atrium, hypothalamus, and kidneys sense changes in vascular pressure. When pressure is low, they stimulate water conservation through AVP release and sodium retention through renin release. When pressure is high, they inhibit AVP and renin release.
- Renin-angiotensin-aldosterone system (RAAS) - the RAAS (see aldosterone / kidney control above) promotes sodium reabsorption along the nephron when it senses reduced renal perfusion, decreased sodium levels, and increased sympathetic tone
- Natriuretic peptides - natriuretic peptides are cardiac proteins released by the atria (ANP) and ventricles (BNP) in response to volume overload and distension. ANP and BNP counteract the effects of renin and AVP in the kidneys, thus promoting the excretion of sodium and water.
- Renal excretion of sodium and water - the kidneys are the primary site of sodium and water regulation. Sodium and water pass freely into the renal tubule, where they are absorbed at different rates along the nephron. Water and sodium tend to move together, but their permeability along the nephron differs, so they are not reabsorbed equally. In the collecting duct, AVP controls water reabsorption independent of sodium. The kidneys are capable of excreting and conserving large amounts of water, producing urine that ranges in osmolality from 50 mOsm/kg to 1200 mOsm/kg.
- Reabsorption of sodium and water along the nephron under normal conditions
- Proximal convoluted tubule: 70% of sodium and 70% of water
- Loop of Henle: 20% of sodium and 10% of water
- Distal convoluted tubule: 6% of sodium and 0% of water
- Collecting ducts: 3% of sodium and 19% of water
HYPONATREMIA
- Overview
- Hyponatremia is defined as a plasma sodium level < 135 mEq/L. It is seen in up to 35% of hospitalized patients and around 2% of outpatients.
- Sodium is a major extracellular solute, so hyponatremia typically leads to serum hypotonicity. Less frequently, it occurs in the setting of serum isotonicity or hypertonicity. In hypotonic hyponatremia, body fluid status is important to consider because it can help identify the underlying disorder. The table below groups causes of hyponatremia by tonicity and volume status. [19,20]
| Hypotonic hyponatremia (serum osmolality < 275 mOsm/kg) |
|---|
|
Hypovolemic causes - total body water decreases more than the decrease in total body sodium
Euvolemic causes - total body water increases with stable total body sodium
Hypervolemic causes - total body water increases more than total body sodium. Water retention is driven by a decrease in effective circulating volume.
|
| Isotonic hyponatremia (serum osmolality 275 - 290 mOsm/kg) |
Pseudohyponatremia
Absorption of solutions used in procedures
Sucrose and maltose in IVIG infusions
|
| Hypertonic hyponatremia (serum osmolality > 290 mOsm/kg) |
Overview
Hyperglycemia
Mannitol
Absorption of solutions used in procedures
Sucrose and Maltose in IVIG infusions
|
- Medications that have been associated with SIADH
- Drugs that stimulate ADH release
- Amitriptyline (Elavil®)
- Carbamazepine (Tegretol®)
- Carboplatin
- Cisplatin
- Cotrimoxazole
- Cyclophosphamide
- Desipramine (Norpramin®)
- Haloperidol (Haldol®)
- Ifosfamide
- Levamisole
- MAO inhibitors
- MDMA "Ecstasy" "Molly" (3,4-methylenedioxymethamphetamine)
- Melphalan
- Methotrexate
- Nicotine
- Opiate medications
- Oxcarbazepine (Trileptal®)
- SSRI antidepressants
- Thioridazine (Mellaril®)
- Tramadol (Ultram®)
- Trifluoperazine (Stelazine®)
- Valproic acid (Depakote®)
- Vinblastine
- Vincristine
- Drugs that enhance the effects of ADH
- Carbamazepine (Tegretol®)
- Chlorpropamide
- Clofibrate
- Cyclophosphamide
- Desmopressin (DDAVP)
- Griseofulvin
- Lamotrigine (Lamictal®)
- NSAIDs
- Oxytocin
- Theophylline
- Tolbutamide
- Other drugs with unclear mechanism
- ACE inhibitors
- Bromocriptine
- Linezolid (Zyvox®)
- Oxytocin
- Venlafaxine [2,19,20,22,50]
- Symptoms
- Symptoms of hyponatremia depend upon the degree and acuteness of sodium decline. Patients with levels > 120 mEq/L typically have no symptoms, while levels below 120 mEq/L may cause nonspecific symptoms such as headache, fatigue, nausea, vomiting, and muscle cramps. When levels fall below 115 mEq/L, brain edema and neurologic decline (e.g. obtundation, seizures, coma) can occur. Rapid declines (< 48 hours) are more likely to be symptomatic than gradual ones. [19,20]
| Steps for evaluating hyponatremia |
|---|
Step 1 - check serum osmolality
|
Step 2 - check urine osmolality
|
|
Step 3 - check a spot urine sodium and/or calculate the fractional excretion of sodium
|
Other tests that may be helpful in select patients
|
- Treatment
- Significant hyponatremia can lead to life-threatening cerebral edema. In most tissues, sodium concentrations in plasma and interstitial fluid are equivalent because sodium passes freely between these compartments. In the brain, capillaries are lined by astrocyte foot processes, which form the blood-brain barrier. The blood-brain barrier is impermeable to sodium which means that brain interstitial fluid is unable to rapidly adjust its osmolality in response to hyponatremia. When serum osmolality drops too quickly, osmosis pulls water into brain tissue, and cerebral edema occurs.
- Treating significant hyponatremia is complicated because rapid corrections of sodium levels can kill astrocytes and lead to osmotic demyelination syndrome (ODS). ODS is a permanent condition, and in severe cases, the patient is left in a vegetative state.
- The recommendations below provide some general guidance on treating hyponatremia. Patients with severe and/or symptomatic hyponatremia should be treated in the intensive care unit. [17,18]
- Hyponatremia levels and acuteness
- Mild hyponatremia: 130 - 135 mEq/L
- Moderate hyponatremia: 125 - 129 mEq/L
- Severe hyponatremia: < 125 mEq/L
- Acute hyponatremia: < 48 hours
- Chronic hyponatremia: ≥ 48 hours or unknown
- Treatment recommendations for symptomatic hyponatremia
- Acute hyponatremia (< 48 hours)
- Patients with acute hyponatremia (< 48 hours) should have their sodium increased by 4 - 6 mEq/L as quickly as possible to prevent brain herniation
- For severe symptoms, give 100 mL of 3% saline infused intravenously over 10 minutes up to 3 times
- For mild to moderate symptoms and low risk for herniation, give 3% NaCl infused at 0.5 - 2 ml/kg/hr
- Chronic hyponatremia (≥ 48 hours or unknown)
- Correct sodium by 4 - 8 mEq/L per day
- Risk factors for ODS include hypokalemia, liver disease, malnutrition, and alcoholism
- For patients at high risk of ODS, correct sodium by 4 - 6 mEq/L per day and do not correct sodium by more than 8 mEq/L in any 24-hour period
- For patients at normal risk for ODS, the maximum correction is 10 - 12 mEq/L in any 24-hour period and 18 mEq/L in any 48-hour period
- The formula below can be used to estimate the change in sodium levels based on the selected infusate
- Calculating sodium replacement
- The amount of sodium infusate needed to raise the serum sodium by the desired level can be calculated with the formula below
- Change in serum Na+ (mEq/L) = [(Infusate Na+ + infusate K+) - serum Na+] / [Total body water + 1]
- Where the estimated total body water in liters is 0.6 L/kg in men and children, 0.50 L/kg in women and elderly men, and 0.45 L/kg in elderly women
- Infusate Na+ is the number of sodium milliequivalents (mEq) in one liter of the infusion
- Infusate K+ is the number of potassium milliequivalents (mEq) in one liter of the infusion
- 3% sodium chloride contains 513 mEq of Na+ per liter
- 0.9% sodium chloride contains 154 mEq of Na+ per liter
- Example:
- Patient is a 40-year-old female that weighs 60 kg with symptomatic hyponatremia and a sodium of 112 mEq/L
- Her provider would like to raise her sodium by 3 mEq/L with an infusion of 3% NaCl
- Using the above formula, 1 liter of 3% NaCl would raise her sodium by 12.9 mEq/L.
- Change in Na+ = [(513 + 0) - 112 / [(0.5 X 60) + 1] = 12.9
- Given that 1 liter of 3% NaCl raises sodium by 12.9 mEq/L, 232 ml would raise sodium by 3 mEq/L (3/12.9 = 0.232 L)
- Her provider orders a 232 ml infusion of 3% NaCl
- Treatment recommendations for SIADH
- In severe cases, fluid restriction with a goal of 500 ml/day below the 24-hour urine output is first-line therapy. If fluid restriction is not successful, consider demeclocycline (blocks effect of ADH in collecting duct), urea (produces osmotic diuresis), or vasopressin receptor antagonists (conivaptan, tolvaptan).
- Infused normal saline is not recommended because the infused sodium is excreted at a greater rate than the infused water and the net effect is the addition of more electrolyte-free water
- In asymptomatic patients, fluid restriction to < 1000 ml/day is recommended, along with addressing the underlying cause if possible (e.g. medications). Loop diuretics (furosemide 20 - 40 mg/day) in combination with increased dietary sodium or 6 - 9 grams of sodium chloride tablets per day (each 1 gram sodium chloride tablet contains 17 mEq of sodium) may also be tried. Demeclocycline 600 - 1200 mg/day, vasopressin receptor antagonists (conivaptan, tolvaptan), and urea may be used in resistant cases but only under close observation by a provider who is familiar with their use. [19,20,21]
- Treatment recommendations for hypovolemic hyponatremia
- Give isotonic saline to correct the volume contraction and hold diuretics. Replace potassium if necessary. [19,20]
- Treatment recommendations for hypervolemic hyponatremia
- Treat hypervolemic hyponatremia with salt and fluid restriction, loop diuretics, and correction of the underlying condition [19,20]
HYPERNATREMIA
- Overview
- Hypernatremia is defined as a plasma sodium level > 145 mEq/L. It is primarily seen in infants and elderly patients because these populations are at risk for inadequate fluid intake. Infants with diarrhea often do not receive proper fluid replacement, and elderly patients can have dementia and other neurologic disorders that impair the thirst mechanism and/or the ability to obtain fluids. In the ICU setting, hypernatremia occurs in up to 26% of patients due to the frequent use of 0.9% NaCl for fluid replacement.
- Hypernatremia occurs when there is a deficit of total body water in relation to the amount of sodium present. Water deficits occur through three primary mechanisms: (1) free water loss (e.g. diabetes insipidus) or unreplaced loss (e.g. impaired thirst), (2) hypotonic fluid loss (e.g. gastroenteritis, nasogastric suctioning), (3) sodium gain (e.g. drinking seawater, hypertonic IV fluids). Most cases of hypernatremia are due to inadequate fluid replacement, which means patients are typically hypovolemic, but it can also occur in euvolemic (e.g. diabetes insipidus) and hypervolemic patients (e.g. renal failure). See sodium regulation illustration above.
- The table below groups causes of hypernatremia by the three main underlying etiologies [24,25,26]
| Causes of hypernatremia |
|---|
|
Free water loss or unreplaced loss
|
|
Hypotonic fluid loss
|
|
Sodium gain
|
- Medications that have been associated with AVP resistance
- Amphotericin B
- Cidofovir
- Cimetidine
- Clozapine
- Colchicine
- Contrast agents
- Cyclophosphamide
- Demeclocycline
- Dopamine
- Epirubicin
- Fluvoxamine
- Foscarnet
- Gentamicin
- Ifosfamide
- Lithium
- Methicillin
- Methoxyflurane
- Ofloxacin
- Orlistat
- Phenytoin (uncommon at therapeutic doses)
- Pimozide
- Propoxyphene
- Rifampin
- Streptozocin
- Vasopressin receptor antagonists (conivaptan, tolvaptan)
- Verapamil
- Vinblastine [2,24,26]
- Symptoms
- Most patients with hypernatremia are also hypovolemic, so they often have symptoms of dehydration (e.g. hypotension, tachycardia). Patients with AVP deficiency or resistance will have polyuria and polydipsia. Infants may display hyperpnea, weakness, restlessness, irritability, insomnia, and lethargy.
- Severe hypernatremia (> 160 mEq/L) and/or hypernatremia with an acute onset (< 24 hours) can cause brain shrinkage. In most tissues, sodium concentrations in plasma and interstitial fluid are equivalent because sodium passes freely between these compartments. In the brain, capillaries are lined by astrocyte foot processes, which form the blood-brain barrier. The blood-brain barrier is impermeable to sodium which means that brain interstitial fluid is unable to rapidly adjust its osmolality in response to hypernatremia. When serum osmolality increases too quickly, osmosis pulls water out of the brain, causing it to shrink. Brain shrinkage can cause blood vessels to tear, leading to hemorrhaging, neurologic damage, and even death.
- Evaluation / Diagnosis
- The etiology of hypernatremia can typically be determined by evaluating the patient's volume status and renal concentrating ability. Patients with AVP deficiency or resistance often do not have hypernatremia but can develop it if access to water is limited. The labs below may be helpful depending on the patient's presentation. [24,25,26]
| Evaluating hypernatremia |
|---|
|
Evaluating patient volume status
|
|
Evaluating renal concentrating ability
|
|
Evaluating for AVP deficiency and resistance (diabetes insipidus)
|
- Treatment
- The treatment of hypernatremia depends upon the acuteness of the condition and the underlying etiology. Treating significant hypernatremia is complicated because lowering sodium levels too rapidly can cause brain swelling and permanent neurologic damage. The recommendations below provide some general guidance. [24,26]
- Symptomatic hypernatremia treatment recommendations
- Acute symptomatic hypernatremia (< 24 hours)
- Hypernatremia that occurs in less than 24 hours (e.g. inadvertent administration of hypertonic fluids) puts the patient at risk for brain shrinkage and vessel rupture. Acute hypernatremia can be corrected more rapidly because the brain has not had adequate time to raise its osmolality.
- The recommended rate of sodium reduction in acute hyponatremia is 1 mEq/L per hour. Some sources recommend a reduction of 2 - 3 mEq/L per hour over 2 - 3 hours while not exceeding 12 mEq/L in 24 hours. [24,26]
- Chronic hypernatremia (> 24 hours or unknown)
- In chronic hypernatremia, brain tissue has adapted to the increase in serum osmolality, so sodium levels should be corrected at a slower pace
- The recommended rate of sodium reduction in chronic hypernatremia is 0.5 mEq/L per hour with a target reduction of 8 - 10 mEq/L over 24 hours [24,26]
- Calculating free water replacement
- The amount of free water needed to lower the serum sodium by a desired level can be calculated with the formula below
- Change in serum Na+ (mEq/L) = [(Infusate Na+ + infusate K+) - serum Na+] / [Total body water + 1]
- Where the estimated total body water in liters is 0.6 L/kg in men and children, 0.50 L/kg in women and elderly men, and 0.45 L/kg in elderly women
- Infusate Na+ is the number of sodium milliequivalents (mEq) in one liter of the infusion
- Infusate K+ is the number of potassium milliequivalents (mEq) in one liter of the infusion
- 5% dextrose in water (D5W) contains no sodium
- 0.2% sodium chloride in D5W contains 34 mEq of Na+ per liter
- 0.45% sodium chloride contains 77 mEq of Na+ per liter
- Ringer's lactate contains 130 mEq of Na+ per liter
- 0.9% sodium chloride contains 154 mEq of Na+ per liter [24]
- Example:
- Patient is a 50-year-old female who weighs 53 kg with a sodium of 159 mEq/L
- Her provider would like to lower her sodium by 6 mEq/L over the next 12 hours with an infusion of 0.45% NaCl
- Using the above formula, 1 liter of 0.45% NaCl would lower her sodium by 2.98 mEq/L
- Change in Na+ = [(77 + 0) - 159 / [(0.5 X 53) + 1] = -2.98
- Given that 1 liter of 0.45% NaCl lowers sodium by about 3 mEq/L, 2 liters would lower sodium levels by 6 mEq/L
- Her provider orders a 0.45% NaCl infusion at a rate of 167 ml/hr over the next 12 hours
- AVP disorder treatment
- AVP deficiency (central diabetes insipidus) - Desmopressin (DDAVP), a synthetic analog of AVP, is recommended for AVP deficiency. Desmopressin comes in a tablet and nasal spray. Adult dosing for the tablet typically starts at 0.05 mg twice daily, with titration based on response to a daily dose of 0.1 - 1.2 mg given in 2 - 3 divided doses. In trials, most patients were maintained on 0.1 - 0.8 mg/day. Adult dosing for DDAVP nasal spray is in the range of 0.1 - 0.4 ml per day given in 1 - 3 divided doses. Most adults can be maintained on 0.1 ml twice daily. [DDAVP tablet PI] [DDAVP nasal spray PI]
- AVP resistance (nephrogenic diabetes insipidus) - AVP resistance is more difficult to treat because synthetic AVP does not work. Certain medications from different drug classes can help lessen water loss by reducing sodium and water delivery to the distal nephron. Thiazide diuretics and amiloride promote sodium loss in the distal nephron, which stimulates sodium and fluid reabsorption in the proximal nephron. The net effect is decreased delivery of water and solutes to the collecting duct. NSAIDs have a similar effect through their inhibition of the RAAS, and a low-sodium diet can enhance these processes. The sulfonylurea chlorpropamide has been shown to potentiate the renal response to AVP. Medications that can worsen AVP resistance should be discontinued (see AVP resistance meds). [26,27]
CHLORIDE
- Overview
- Chloride is the main extracellular anion, and together with bicarbonate, it helps to maintain electroneutrality with the predominant cation, sodium. Chloride and bicarbonate levels often move in opposite directions because they replace each other when one is lost so that electroneutrality is preserved. Because of its association with bicarbonate, chloride levels are affected by acid-base disorders. Unlike sodium and potassium, chloride homeostasis is not managed directly. Instead, it is regulated passively through sodium and bicarbonate adjustments.
- In the nephron, 65 - 70% of chloride is passively reabsorbed in the proximal tubule, 25% is actively reabsorbed in the Loop of Henle, and the remainder is reabsorbed in the distal tubule. Chloride typically follows sodium and water along the nephron, but acid-base disorders that affect bicarbonate excretion can modify this relationship. (see metabolic alkalosis and nephron illustration) [2,3,4]
- Hypochloremia
- Hypochloremia, defined as a chloride level < 98 mEq/L in adults, may occur through excessive chloride loss, acid-base disorders, or extracellular fluid expansion. It is primarily treated by correcting the underlying disorder. If chloride replenishment is necessary, it can be achieved with one of its salt forms, typically sodium chloride or potassium chloride. The choice of salt will depend upon the underlying disorder.
- Some causes of hypochloremia are listed below
- Excessive chloride loss
- Vomiting - gastric fluid contains 1.5 - 3 times more chloride than sodium
- Nasogastric suctioning
- Diarrhea
- Excessive sweating
- Diuretics - through direct chloride loss and metabolic alkalosis
- Intrinsic kidney disease (e.g. salt-losing nephropathy)
- Hyperaldosteronism - aldosterone stimulates bicarbonate retention in the distal nephron, and chloride is lost
- Cushing's syndrome - through excessive mineralocorticoid activity
- Corticosteroids - through excessive mineralocorticoid activity
- Bartter and Gitelman syndrome - rare disorders that decrease the function of the Na-Cl transporter in the distal nephron
- Acid-base disorders
- Metabolic alkalosis - metabolic alkalosis is typically driven by chloride loss and/or excessive mineralocorticoid activity
- Diuretics - through direct chloride loss and metabolic alkalosis
- Chronic respiratory acidosis - promotes the retention of bicarbonate in the kidneys, and chloride is lost
- High anion gap acidosis - bicarbonate retention in the kidneys causes chloride loss, and excessive anion displaces chloride in the extracellular fluid
- Extracellular fluid expansion
- Primary polydipsia
- SIADH
- Hypotonic fluid infusion (e.g. D5W) [2,3,4]
- Hyperchloremia
- Hyperchloremia, defined as a chloride level > 110 mEq/L in adults, may occur through excessive chloride gain, decreased chloride excretion, acid-base disorders, and extracellular fluid contraction. Hyperchloremia is treated by addressing the underlying condition.
- Some causes of hyperchloremia are listed below
- Excessive chloride gain
- Hypertonic saline fluids (e.g. 3% NaCl, swallowing seawater)
- Excessive normal saline
- Normal saline with KCl
- Total parenteral nutrition
- Decreased chloride excretion
- Kidney failure
- Renal tubular acidosis
- Nephrotic syndrome
- Acid-base disorders
- Normal anion gap metabolic acidosis - bicarbonate loss causes chloride retention
- Respiratory alkalosis - excessive CO2 loss causes bicarbonate excretion and chloride retention
- Extracellular volume contraction
- Dehydration
- Diabetes insipidus [2,3,4]
CALCIUM AND PHOSPHORUS HOMEOSTASIS
- Overview
- Calcium and phosphorus (also called phosphate) are closely related and regulated by the same hormones, mainly parathyroid hormone and calcitriol (1,25-dihydroxyvitamin D)
- The human body contains about 1000 grams of calcium, and 99% of it is found in bones. While most calcium is utilized to harden bones, the remaining 1% plays an important role in muscle contraction, nerve conduction, and blood clotting (calcium is factor IV in the coagulation cascade). Low calcium levels increase the excitability of neurons and can lead to tetany (sustained muscle contractions). High calcium levels depress nerve activity and can lead to lethargy, constipation, and weakness.
- The human body contains about 1000 grams of phosphorus, and 85% of it is found in bones, where it combines with calcium to form hydroxyapatite, a mineral that provides strength. The remaining 15% is found mostly in cells, making it a major intracellular anion. Intracellular phosphorus is involved in a number of important physiologic processes, including protein, lipid, and carbohydrate metabolism and the formation of high-energy bonds in ATP. It also acts as an acid-base buffer and is part of many cellular components, including phospholipid membranes, RNA, DNA, and messenger proteins. [2,3,4,34]
- Calcium and phosphorus regulation
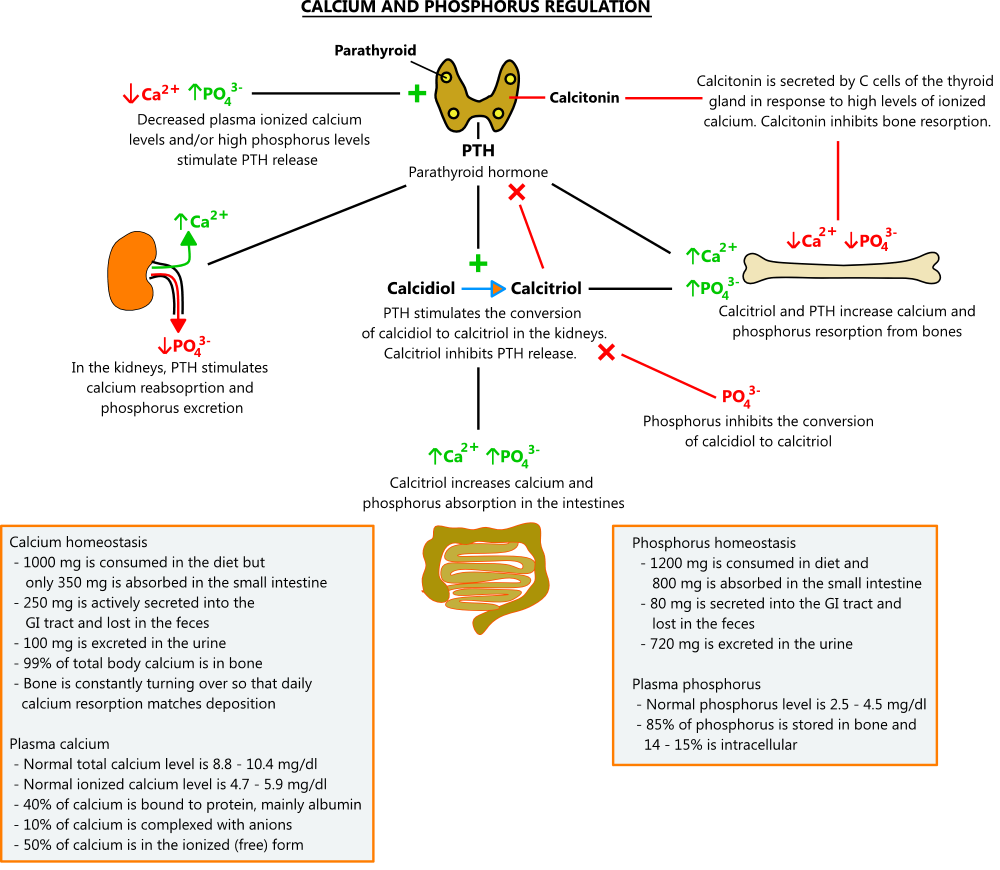
- Calcium
- Because of its role in nerve conduction and muscle contraction, calcium levels must be tightly regulated. The main hormones that control calcium levels are parathyroid hormone (PTH) and calcitriol. Calcitonin plays a role too, but in adults, its actions have a minimal effect; even after thyroidectomy, when calcitonin levels are unmeasurable, calcium regulation remains intact.
- PTH regulates calcium levels through a number of mechanisms. When calcium levels are low, PTH secreted from the parathyroid glands stimulates bone resorption, causing the release of calcium and phosphorus. In the kidneys, it promotes the reabsorption of calcium, excretion of phosphorus, and conversion of calcidiol to its active form, calcitriol. Calcitriol enhances bone resorption and stimulates calcium and phosphorus uptake from the small intestine. When calcium levels are high, PTH secretion is inhibited, and these processes are suppressed.
- In plasma, about 50% of calcium circulates in the free ionized form, which is the active form. Ten percent is complexed with anions, and the remaining 40% is bound to protein, mainly albumin. If albumin levels are low, total calcium levels will not reflect the true amount of active calcium. In these cases, ionized calcium levels can be measured directly, or total calcium can be estimated using the formula below.
- Corrected calcium (mg/dL) = measured total Ca (mg/dL) + 0.8 (4.0 - serum albumin [g/dL])
- where 4 represents the normal albumin level
- Acid-base disorders can also affect calcium protein binding; alkalosis increases binding, and acidosis decreases it [2,3,4,32,34]
- Phosphorus
- In bone, phosphorus combines with calcium to form hydroxyapatite, a mineral that provides strength. In extracellular fluid, calcium and phosphorus are prohibited from binding by chemical inhibitors. If phosphorus levels get too high (e.g. kidney disease), the inhibitors can become overwhelmed, allowing calcium phosphate to form in soft tissues. This can lead to cardiac and respiratory failure. Conversely, if phosphate levels are too low, bone mineralization is impaired, and osteomalacia can occur.
- High serum phosphorus can bind calcium and lower ionized levels, stimulating PTH release. Although PTH promotes processes that increase phosphorus levels (e.g. bone resorption, calcitriol production), these effects are overcome by its ability to enhance renal phosphorus excretion. In addition, high phosphorus levels directly inhibit the conversion of calcidiol to calcitriol. The net effect is reduced phosphorus. When phosphorus levels are low, these processes are suppressed. [2,3,4]
HYPOCALCEMIA
- Overview
- The lower limit of normal for serum calcium levels varies by lab, but in general, hypocalcemia in adults is defined as a total calcium level < 8.8 mg/dl (ionized calcium < 4.7 mg/dl). The incidence of hypocalcemia in the general population is not well-defined; the most common causes are acute and chronic renal failure, vitamin D deficiency, magnesium deficiency, and acute pancreatitis. After thyroidectomy and parathyroid surgery, transient and permanent hypocalcemia are seen in about 30% and 1 - 2% of patients, respectively. [32,34,35]
| Causes of hypocalcemia |
|---|
|
- Medications that have been associated with hypocalcemia
- Bisphosphonates (e.g. Fosamax, Actonel)
- Calcitonin
- Carbamazepine - carbamazepine can increase vitamin D metabolism through CYP induction
- Cinacalcet - cinacalcet is a calcimimetic agent used to treat secondary hyperparathyroidism in dialysis patients
- Cisplatin - causes hypomagnesemia
- Citrated blood or plasma products - citrate binds calcium. Patients with hepatic or renal failure who are slow to clear citrate are at the greatest risk.
- Denosumab (Prolia®)
- Estrogens - estrogens inhibit bone resorption and may worsen hypocalcemia
- Ethanol - inhibits PTH release
- Ethylenediaminetetraacetic acid (EDTA) - chelating agent
- Fluoride
- Foscarnet
- Gadolinium-based contrast agents (e.g. gadodiamide, gadoversetamide) - interfere with calcium assays and can cause false-low readings
- H-2 blockers (e.g. famotidine) - higher gastric pH may reduce calcium and magnesium absorption
- Leucovorin
- Nivolumab
- Pembrolizumab
- Phenobarbital - phenobarbital can increase vitamin D metabolism through CYP induction
- Phenytoin - phenytoin can increase vitamin D metabolism through CYP induction
- Proton pump inhibitors (e.g. omeprazole) - higher gastric pH may reduce calcium and magnesium absorption
- Sodium phosphate products (Fleet® enema) - may cause hyperphosphatemia in susceptible populations (e.g. renal or hepatic failure, CHF)
- 5-fluorouracil [32,34,35]
- Symptoms
- Symptoms of hypocalcemia depend upon the magnitude of the deficit. Mild hypocalcemia is typically asymptomatic and often discovered incidentally on routine labs. Significant hypocalcemia increases the excitability of neurons, causing paresthesias, muscle cramps, tetany, and in severe cases, seizures. Cardiac changes, including reduced contractility and prolonged QT interval, can also occur. Two classic physical exam findings, Chvostek's and Trousseau's signs, are associated with hypocalcemia. Chvostek's sign is a facial twitch elicited by tapping on the facial nerve just below the zygomatic bone with the patient's mouth slightly open. Trousseau's sign is induced by inflating a blood pressure cuff to 20 mmHg above systolic blood pressure for 3 - 5 minutes. This occludes the brachial artery, and the neuromuscular irritability induced by hypocalcemia causes wrist and metacarpophalangeal flexion, interphalangeal joint extension, and thumb adduction (see Trousseau's sign illustration). [32,34,35]
| Steps for evaluating hypocalcemia |
|---|
Step 1 - Confirm that hypocalcemia is real
|
|
Step 2 - Depending on the patient, consider the following:
|
- Treatment
- Hypocalcemia is primarily treated by addressing the underlying disorder. Severe, symptomatic hypocalcemia should be treated with intravenous calcium. Treatment recommendations for common etiologies are reviewed below.
- Severe hypocalcemia (< 7.5 mg/dl)
- Severe, symptomatic hypocalcemia requires prompt treatment. Most patients are treated with calcium gluconate (10 ml of 10% solution contains 94 mg of elemental calcium), which can be given as an initial bolus (100 - 300 mg over 5 - 10 minutes) followed by a slow infusion. Calcium chloride (10 ml of 10% solution contains 272 mg of elemental calcium) contains more calcium but must be given through a central line. After an initial bolus, calcium can be infused at a rate of 0.5 mg/kg/hr and increased to 2 mg/kg/hr as needed. Patients on calcium infusions should be monitored under telemetry. [32,34,35]
- Chronic kidney disease
- Recommendations for monitoring and treating hypocalcemia and hyperparathyroidism from the National Kidney Foundation are presented below
- Monitoring
- CrCl 15 - 29 ml/min: check serum calcium and phosphate every 3 – 6 months and PTH every 6 – 12 months
- CrCl < 15 ml/min: check serum calcium and phosphate every 1 - 3 months and PTH every 3 - 6 months
- In both groups, check 25-hydroxyvitamin D level to screen for deficiency. Consider BMD in high-risk groups.
- Bone-specific alkaline phosphatase can also be used to screen for bone disease [36]
- Hyperphosphatemia treatment
- The 2017 NKF guidelines state that decisions about phosphate-lowering treatment should be based on progressively or persistently elevated serum phosphate and not on one reading. The goal of phosphate-lowering therapy is to lower phosphate levels toward the normal range. The guidelines do not specify when therapy should be initiated or which drug is preferred. Treatment options are reviewed below. [36]
- Low-phosphate diet - all patients with hyperphosphatemia should consume a low-phosphate diet. The typical Western diet contains 1000 - 1200 mg of phosphorus per day. Restricting phosphorus intake to less than 800 mg a day can help lower levels. Information on phosphate-restricted diets is available at the links below.
- Calcium carbonate (Tums®, Os-Cal®, Caltrate®) - calcium binds phosphate in the intestine and prevents its absorption. Calcium carbonate contains 40% elemental calcium, so 500 mg of calcium carbonate will have 200 mg of elemental calcium (0.40 X 500 mg). A typical starting dose for hyperphosphatemia is 200 mg of elemental calcium (500 mg of calcium carbonate) with each meal. Calcium carbonate doses should not exceed 3750 mg/day (1500 mg elemental calcium) so that total daily calcium intake does not exceed 2000 mg. With calcium carbonate therapy, average phosphorus levels decline by 0.9 mg/dl, and average calcium levels increase by 0.5 mg/dl. Calcium carbonate is inexpensive and has been used for many years. Its primary disadvantage is that it increases hypercalcemia risk.
- Calcium acetate (PhosLo®, Eliphos®) - calcium binds phosphate in the intestine and prevents its absorption. Calcium acetate is available by prescription in 667 mg tablets and capsules. Each capsule contains 169 mg of elemental calcium. The recommended starting dose is 2 pills with each meal, and doses above 9 pills/day should be used with caution. In a study from the PhosLo PI, an average of 3.4 tablets per meal lowered phosphorus levels by 2.4 ml/dl and increased calcium levels by 0.8 mg/dl over 12 weeks. Calcium acetate is more expensive than calcium carbonate but cheaper than other therapies. [PhosLo PI]
- Sevelamer (Renagel®, Renvela®) - sevelamer is an anion exchange resin that binds phosphorus in the intestine and prevents its absorption. Renagel is sevelamer hydrochloride, and Renvela is sevelamer carbonate. Sevelamer carbonate was developed over concerns that the hydrochloride version could contribute to metabolic acidosis. The starting dose is 800 mg (phosphorus 5.5 - 7.4 mg/dl) to 1600 mg (phosphorus ≥ 7.5 mg/dl) three times a day, followed by titration to the desired effect. In studies, sevelamer reduced the average phosphorus level by 2 mg/dl over 52 weeks. The main advantage of sevelamer is that it does not raise calcium levels. Side effects are mainly gastrointestinal and include vomiting (22%), nausea (20%), diarrhea (19%), and dyspepsia (16%). [Renvela PI]
- Lanthanum (Fosrenol®) - lanthanum binds phosphorus in the intestines and prevents its absorption. Lanthanum comes as a chewable tablet in doses of 500, 750, and 1000 mg. The recommended starting dose is 500 mg three times daily with meals followed by titration to the desired effect. In one trial, lanthanum (2250 mg/day) reduced phosphate levels by 2 mg/dl over 6 weeks. Advantages of lanthanum are that it does not raise calcium levels and it has a generic version. [Fosrenol PI] A study that compared lanthanum to calcium carbonate for hyperphosphatemia in patients undergoing dialysis found no difference in the risk of cardiovascular events between the two treatments. [PMID 34003226]
- Sucroferric oxyhydroxide (Velphoro®) - sucroferric oxyhydroxide is an iron-based compound that binds phosphorus in the intestines and prevents its absorption. It comes in a 500 mg chewable tablet, and the recommended starting dose is 500 mg three times a day with meals, followed by titration to the desired effect. In one trial, Velphoro reduced phosphorus levels by an average of 2.2 mg/dl over 12 weeks. [PMID 24646861] Velphoro is expensive, and each tablet contains 500 mg of iron, so it should not be used in patients with iron overload (e.g. hemochromatosis). [Velphoro PI]
- Ferric citrate (Auryxia®) - ferric citrate is an iron-based compound that binds phosphorus in the intestines and prevents its absorption. Ferric citrate comes in a 210 mg tablet, and the recommended starting dose is 2 tablets three times a day with meals. The dose can be titrated by 1 - 2 tablets a day at a minimum of 1-week intervals to a maximum of 12 tablets/day. In a trial from the Auryxia PI, Auryxia reduced serum phosphorus levels by about 2 mg/dl over 52 weeks. Auryxia has 210 mg of ferric iron in each tablet and is also approved to treat anemia in CKD. Auryxia has no generic and is very expensive. [Auryxia PI] [36,37]
- Tenapanor (Xphozah®) - Tenapanor inhibits sodium/hydrogen exchanger 3 (NHE3) in intestinal epithelial cells, reducing sodium and phosphate absorption. Diarrhea is its main side effect, affecting up to 53% of patients. In studies, tenapanor lowered phosphate levels by 0.7 mg/dl compared to placebo. The recommended dose is one 30 mg tablet twice daily before meals. Tenapanor has no generic and is very expensive. [Xphozah PI]
- Hyperparathyroidism treatment
- The 2017 NKF guidelines state that the optimal PTH level in adult patients with kidney disease who are not on dialysis is unknown. Patients with progressively rising or persistently elevated PTH levels should be evaluated for hyperphosphatemia, hypocalcemia, high phosphate intake, and vitamin D deficiency. Calcitriol and vitamin D analogs may be reasonable in patients with severe and progressive hyperparathyroidism, but non-dialysis patients should not routinely receive them.
- Calcitriol, doxercalciferol, and paricalcitol are prescription vitamin D analogs that differ from other vitamin D supplements (e.g. ergocalciferol, cholecalciferol) in that they do not require kidney metabolism to become active. A brief review of each drug is provided below. It is important that patients maintain adequate calcium intake while taking these drugs; depending on the individual, combined dietary and supplemental calcium intake should range between 600 - 1200 mg/day. Frequent lab monitoring is required to prevent hypercalcemia. See vitamin D supplements and calcium supplements for more information on available products. [36]
- Calcitriol (Rocaltrol®; 1,25 dihydroxyvitamin D) - calcitriol is the biologically active form of vitamin D (see vitamin D metabolism). The recommended starting dose in predialysis patients is 0.25 mcg/day, with an increase to 0.5 mcg/day if necessary. [Rocaltrol PI]
- Doxercalciferol (Hectorol®) - doxercalciferol is a synthetic vitamin D2 analog. Doxercalciferol is converted in the liver to 1α,25-(OH)2D2, an active metabolite that does not require kidney metabolism. The recommended starting dose in predialysis patients is 1 mcg once daily. The dose may be increased by 0.5 mcg/day at 2-week intervals to a maximum of 3.5 mcg once daily. [Hectorol PI]
- Paricalcitol (Zemplar®) - paricalcitol is a synthetic version of calcitriol that does not require metabolism to become active. The recommended starting dose in predialysis patients with a GFR of 15 - 60 ml/min is 1 mcg once daily if the PTH level is < 500 pg/ml and 2 mcg once daily if it is > 500 pg/ml. Dosing is then adjusted based on follow-up PTH levels. [Zemplar PI]
- Hypoparathyroidism
- Post-thyroidectomy and parathyroidectomy
- Transient hypocalcemia is common after thyroidectomy and parathyroid surgery. Severe cases may require parenteral calcium, but most patients can be managed with oral calcium and vitamin D. Most surgeons have a calcium protocol they follow starting on the day of surgery. A typical regimen calls for 200 - 600 mg of daily elemental calcium tapered over 2 - 3 weeks to a maintenance dose of 100 - 200 mg/day. Food increases the absorption of calcium carbonate but does not affect calcium citrate. Supplementary vitamin D should be included to ensure an intake of at least 600 IU/day. A paper that outlines one such protocol is available here - PMID 20610248.
- In resistant cases, calcitriol may be necessary. The recommended starting dose in hypoparathyroidism is 0.25 mcg/day, titrated every 2 - 4 weeks to a maximum of 2 mcg/day with frequent monitoring of calcium levels. [35,38]
- Other causes
- Patients with hypoparathyroidism from other causes (e.g., idiopathic, autoimmune) are generally treated with calcium and vitamin D supplements. If these treatments are inadequate, a recombinant version of parathyroid hormone called Natpara is available. Natpara has been shown to increase the risk of osteosarcoma in rats, and therefore, its use should be reserved for patients who do not respond to other treatments. It is given as a once-daily subcutaneous injection. [Natpara PI] In 2024, another parathyroid hormone analog, palopegteriparatide (Yorvipath®), was FDA-approved to treat hypoparathyroidism. [Yorvipath PI] [35]
- Hypomagnesemia
- See hypomagnesemia below
HYPERCALCEMIA
- Overview
- Normal calcium levels vary by lab, but in general, hypercalcemia in adults is defined as a total calcium > 10.4 mg/dl and an ionized calcium > 5.9 mg/dl. The incidence of hypercalcemia in the general population is about 1 - 2%, with hyperparathyroidism and malignancy accounting for up to 90% of cases. Causes of hypercalcemia can generally be broken down into PTH disorders, bone disorders, vitamin D disorders, and other causes. [28,32,33]
| Causes of hypercalcemia |
|---|
|
PTH disorders
|
|
Bone disorders
|
|
Vitamin D disorders
|
|
Other
|
| Hypercalcemia levels | ||
|---|---|---|
| Degree | Total calcium (mg/dl) |
Ionized calcium (mg/dl) |
| Mild | 10.5 - 11.9 | 5.6 - 7.9 |
| Moderate | 12 - 13.9 | 8 - 9.9 |
| Severe | ≥ 14 | ≥ 10 |
- Symptoms
- Symptoms of hypercalcemia depend upon the degree of elevation and the rapidity of onset. Hypercalcemia from malignancy usually presents more acutely and progresses rapidly. Hypercalcemia in primary hyperparathyroidism tends to have an insidious onset and may go undiagnosed for years. Mild hypercalcemia is typically asymptomatic and discovered incidentally on routine labs. When calcium levels rise above 12 mg/dl, symptoms that coined the mnemonic "stones, bones, abdominal moans, and psychic groans" may appear. Hypercalcemia can slow nerve conduction causing lethargy, depression, and confusion. Muscle contractions are also suppressed, leading to constipation and weakness. Prolonged hypercalcemia can cause kidney stones and bone demineralization. Elevated calcium levels have direct effects on the kidneys that include diabetes insipidus, increased sodium excretion, renal vasoconstriction, and parenchymal calcium deposition that can induce kidney failure. In moderate-to-severe hypercalcemia, cardiac conduction abnormalities can occur, including prolonged PR interval, short QT interval, widened QRS complex, bradycardia, and arrhythmias. Levels > 15 mg/dl are considered a medical emergency. [28,32,33]
| Steps for evaluating hypercalcemia |
|---|
Step 1 - Recheck calcium and review medications
|
Step 2 - Evaluate for hyperparathyroidism (> 50% of cases)
If the PTH level is low or low-normal or the above testing is not definitive, proceed to Step 3 |
|
Step 3 - Evaluate for malignancy (33 - 50% of cases)
If the above testing is not definitive, continue to Step 4 |
|
Step 4 - Look for less common causes
|
- Treatment
- Hypercalcemia is primarily treated by correcting the underlying disorder. Treatments used in select etiologies are reviewed below.
- Severe hypercalcemia (> 14 mg/dl)
- Severe hypercalcemia (> 14 mg/dl) should be addressed promptly with aggressive normal saline infusions, the initiation of an intravenous bisphosphonate, and, if necessary, calcitonin. A typical fluid regimen for hypercalcemia consists of a 1 - 2 liter bolus of normal saline followed by an infusion of 100 - 150 ml/hour titrated to achieve a urine output of 100 ml/hour. Patients without comorbidities (e.g. heart failure, renal failure) may be able to tolerate more, and vice versa. [29]
- Primary hyperparathyroidism
- About 85% of cases are caused by a single adenoma. Surgical removal of the affected gland(s) is recommended in all symptomatic patients. In asymptomatic patients, some experts recommend surgery while others only recommend surgery if one of the following criteria is met:
- Serum calcium > 1 mg/dl above the upper limit of normal
- Osteoporosis on BMD or history of vertebral fracture
- GFR < 60 ml/min
- 24-hour urine calcium > 400 mg/day or presence of kidney stones or nephrocalcinosis
- Age < 50 years
- If total parathyroidectomy is performed, a small amount of parathyroid tissue is autotransplanted to the forearm so that calcium regulation is maintained. Additional tissue is also cryopreserved in case the initial transplant does not function properly.
- Transient post-surgical hypocalcemia is seen in about 33% of patients, and permanent hypocalcemia occurs in 2%. See hypocalcemia treatment after parathyroidectomy for more. Recurrent hyperparathyroidism occurs in about 10% of cases. [30]
- Malignancies
- Malignancy-induced hypercalcemia cannot always be cured, but it can be treated with the medications below.
- Bisphosphonates - bisphosphonates inhibit osteoclasts, reducing calcium resorption from bones. Pamidronate and zoledronic acid are intravenous bisphosphonates FDA-approved to treat hypercalcemia. In one study (N=275), pamidronate was compared to zoledronic acid in patients with hypercalcemia of malignancy (average calcium 14 mg/dl). Calcium lowering was seen within 48 hours with both drugs, and normalization of calcium levels occurred in most patients within 4 - 10 days. Duration of response was longer with zoledronic acid (median 32 days vs 18 days). [PMID 11208851] Oral bisphosphonates have not been studied extensively in malignancy-induced hypercalcemia. [Pamidronate PI] [Zoledronic acid PI]
- Denosumab (Xgeva®) - denosumab inhibits RANKL, a ligand that stimulates osteoclast activity (see RANKL illustration). Denosumab is FDA-approved to treat hypercalcemia of malignancy refractory to bisphosphonates. In a study of patients who failed bisphosphonates, 64% of patients treated with denosumab achieved normal calcium levels by 10 days, and the median duration of response was 104 days. [PMID 24915117] [Xgeva PI]
- Calcitonin - calcitonin reduces serum calcium within hours, but its effect wanes after 3 days. It is often used to lower calcium acutely while bisphosphonates, which take several days to work, are being initiated. See calcitonin for more.
- Corticosteroids - corticosteroids, which inhibit the conversion of calcidiol to calcitriol, can help lower calcium in conditions where calcitriol is elevated (e.g. granulomatous diseases) [28,29,33]
- Granulomatous diseases
- Granuloma macrophages possess the 25-hydroxyvitamin D–1α-hydroxylase enzyme that converts calcidiol to its more active form, calcitriol. Elevated calcitriol levels increase calcium absorption and can lead to hypercalcemia. Corticosteroids inhibit 25-hydroxyvitamin D–1α-hydroxylase, suppressing calcitriol production in granulomas. For patients with sarcoidosis, hydroxychloroquine may also be used. [28,29]
PHOSPHORUS
- Overview
- Phosphorus is a major intracellular anion involved in a number of physiologic reactions. In the extracellular fluid, it is only present in small amounts, and it does not play a significant role in tonicity, electrochemical gradients, or acid-base disorders, so abnormal levels are typically asymptomatic. In bones, phosphorus combines with calcium to form hydroxyapatite, a mineral that provides strength, and chronic deficiencies can lead to osteoporosis. Calcium and phosphorus are regulated by the same hormones, so calcium disorders will often affect phosphorus too. (see calcium and phosphorus regulation)
- Abnormal phosphate levels are treated by correcting the underlying disorder. Causes of low and high phosphorus are discussed below. [2,3,4,40]
- Hypophosphatemia
- Hypophosphatemia in adults is defined as a phosphate level < 2.5 mg/dl. Hypophosphatemia typically occurs through one of three mechanisms: (1) inadequate absorption, (2) increased renal excretion, or (3) shift from extracellular to intracellular space.
- Inadequate absorption
- Phosphorus is found in most foods, so inadequate dietary intake is rare. Phosphorus is primarily absorbed in the proximal small intestine, and GI disorders or medications affecting this area can reduce its uptake. Factors that can decrease phosphorus absorption include the following:
- Chronic diarrhea
- Antacids - calcium, aluminum, and magnesium in antacids bind phosphorus in the intestine and prevent its absorption
- Phosphate-binding agents - see treatment of hyperphosphatemia above
- Vitamin D deficiency - vitamin D has a mild stimulatory effect on phosphorus absorption, and in extreme deficiencies, hypophosphatemia can occur
- Increased renal excretion
- Hyperparathyroidism - PTH stimulates phosphorus excretion
- Primary renal phosphate-wasting syndromes - rare genetic disorders that affect renal phosphate reabsorption (e.g. X-linked hypophosphatemic rickets)
- Fanconi syndrome - syndrome marked by proximal renal tubular damage that hinders the reabsorption of glucose, phosphates, and other substances. It may be hereditary or acquired from conditions such as multiple myeloma, Wilson disease, and certain medications (e.g. tetracyclines).
- Increased diuresis - increased diuresis from diuretics, diabetes insipidus, and osmotic agents (e.g. glucose, mannitol) can cause phosphate loss
- Shift from extracellular to intracellular space
- Refeeding syndrome - refeeding syndrome occurs when a person who has been starved for a period is fed. The sudden increase in insulin and glucose stimulates cellular metabolism, which drives phosphate uptake by cells.
- Treatment of diabetic ketoacidosis - similar to refeeding syndrome, the sudden increase in insulin stimulates cellular metabolism, driving phosphate uptake by cells
- Hyperventilation - respiratory alkalosis induced by hyperventilation can cause hypophosphatemia in extreme cases. The process is driven by intracellular phosphate trapping that occurs when carbon dioxide shifts out of cells, raising intracellular pH.
- Hungry bone syndrome - after surgical correction of hyperparathyroidism, a sudden decrease in PTH levels can cause bones to undergo rapid remodeling. These "hungry" bones take up lots of phosphorus, which can lead to hypophosphatasia. [2,41,42]
- Hyperphosphatemia
- Hyperphosphatemia in adults is defined as a phosphate level > 4.5 mg/dl. It typically occurs through one of three mechanisms: (1) decreased renal phosphate excretion, (2) excessive phosphate load, or (3) phosphate shift from intracellular to extracellular space.
- Decreased renal excretion
- Chronic kidney disease - kidney disease is by far the most common cause of hyperphosphatemia. Phosphate levels typically begin to rise when the GFR falls below 30 ml/min. See hyperphosphatemia treatment in CKD for more.
- Tumoral calcinosis - tumoral calcinosis is a rare syndrome marked by calcium salt deposition in periarticular tissue and a deficiency of active fibroblast growth factor 23, a hormone that promotes renal phosphate excretion
- Hypoparathyroidism - loss of PTH-enhanced renal phosphate excretion can cause hyperphosphatemia
- Pseudohypoparathyroidism - rare syndrome where defective PTH receptors do not respond to PTH
- Excessive phosphate load
- Tumor lysis syndrome - chemotherapy-induced cell lysis causes the release of large amounts of intracellular phosphate. Condition is mostly seen with lymphomas and leukemias.
- Rhabdomyolysis - phosphate release from dying muscle tissue
- Severe hemolysis - phosphate release from red blood cells
- Excessive phosphate ingestion - typically from phosphate-containing laxatives
- Hypervitaminosis D - vitamin D stimulates intestinal phosphate absorption
- Shift from intracellular to extracellular space
- Insulin deficiency (e.g. diabetic ketoacidosis)
- Acute acidosis (e.g. lactic acidosis) [2,39,40]
- Evaluation / Diagnosis
- The etiology of abnormal phosphate levels is typically evident from the patient history and other laboratories. In rare cases, a 24-hour urine collection for phosphorus may be required to evaluate renal phosphate excretion. The fractional excretion of phosphate can also be determined from spot urine values with the following formula:
- Fractional excretion of phosphate (FEPO4) = (UPO4 x PCr x 100) / (PPO4 x UCr)
- Where UPO4 is urine phosphate, PCr is plasma creatinine, PPO4 is plasma phosphate, and UCr is urine creatinine
- In hypophosphatemia, a 24-hour urine phosphate excretion of < 100 mg or a FEPO4 < 5% indicates renal phosphate retention and suggests decreased phosphate absorption and/or intracellular shifts. A 24-hour urine excretion of > 100 mg or a FEPO4 > 5% indicates renal phosphate wasting, which is seen in hyperparathyroidism and renal tubular dysfunction.
- In hyperphosphatemia, a FEPO4 < 15% suggests renal failure or hypoparathyroidism. A FEPO4 > 15% indicates excessive phosphate load. [39,40,41,42]
- Treatment
- Hypophosphatemia
- Hypophosphatemia is primarily treated by correcting the underlying disorder. Some general treatment points are provided below.
- Dietary phosphorus - phosphorus is found in most foods, so patients with normal dietary phosphate absorption should consume a regular diet. Foods high in phosphorus include dairy products, meats, and beans. In most patients, consuming 1000 - 2000 mg of phosphorus per day for 7 - 10 days will replenish stores.
- Phosphorus supplements - In some cases (e.g. phosphate-wasting syndromes), a phosphorus supplement may be indicated. Supplements like K-Phos Neutral® come in sodium and potassium salts. K-Phos Neutral® contains 250 mg of phosphorus per tablet, and the recommended intake is 4 - 8 tablets/day with meals. [K-Phos N Neutral PI]
- Vitamin D - vitamin D stimulates intestinal phosphate absorption and promotes renal phosphate conservation; therefore, a vitamin D supplement can help increase phosphorus levels
- Severe hypophosphatemia or unable to absorb phosphorus - for patients who are unable to absorb phosphorus or have severe hypophosphatemia, IV phosphorus is indicated
- Hypophosphatemic rickets - some cases of hypophosphatemic rickets are caused by a genetic mutation in fibroblast growth factor 23 (FGF23), a protein that promotes renal phosphorus excretion. In hypophosphatemic rickets, mutated FGF23 is resistant to metabolism, and its activity is increased. A monoclonal antibody to FGF23 called burosumab (Crysvita®) is FDA-approved to treat this condition. It is also approved to treat tumor-induced osteomalacia. [Crysvita PI] [40]
- Hyperphosphatemia
- Hyperphosphatemia is treated by correcting the underlying disorder. The most common cause of hyperphosphatemia is chronic kidney disease (see treatment of hyperphosphatemia in CKD for more). [40]
MAGNESIUM
- Overview
- Magnesium, the second most abundant intracellular cation, is an important cofactor in numerous physiologic processes, including metabolism, nerve conduction, receptor activation, and the stabilization of cellular components (e.g. RNA, DNA, cell membrane). Magnesium content in the average adult is about 25 grams, with 60% located in bones, 39% inside of cells, and 1% in the extracellular fluid. Sixty percent of extracellular magnesium is in the ionized (free) form, and 40% is bound to proteins and anions. The average American diet contains 360 mg of magnesium per day, but only 33% is absorbed; when stores are depleted, absorption can more than double. The kidneys are the primary regulators of magnesium balance, capable of rapid excretion under high loads and almost total retention during deficits. Unlike other electrolytes, magnesium levels do not influence any particular regulatory hormone. [4,43,44]
- Hypomagnesemia
- Hypomagnesemia is defined as a magnesium level < 1.5 mg/dl. It is present in about 2% of the general population and up to 20% of hospitalized patients. Alcoholics are at greatest risk, with a prevalence of up to 80% in some studies. Causes of hypomagnesemia can be divided into three categories: (1) decreased intake/absorption, (2) abnormal renal loss, (3) shift from extracellular to intracellular space.
- Decreased intake/absorption
- Alcoholics (up to 80%) - from decreased intake
- Proton pump inhibitors (e.g. Prilosec, Nexium) - prolonged PPI use (> 3 months) can reduce magnesium absorption
- Starvation
- Total parenteral nutrition
- Diarrhea
- Vomiting
- Gastric bypass surgery
- Ileostomy - loss of magnesium absorption in the large intestine
- Nasogastric suctioning
- Hypomagnesemia with secondary hypocalcemia - rare genetic disorder that causes nonfunctional magnesium channels in the intestine and kidneys
- Cystic fibrosis - possibly through decreased intestinal absorption and/or renal wasting
- Abnormal renal loss
- Diuretics (most common) - greatest risk is with loop diuretics because they alter electrochemical gradients in the Loop of Henle, where most magnesium is reabsorbed. May also occur with chronic thiazide therapy and osmotic diuretics.
- Antimicrobials - amphotericin B, aminoglycosides, pentamidine, capreomycin, viomycin, foscarnet
- Chemotherapeutic agents - cisplatin, cetuximab
- Immunosuppressants - tacrolimus, cyclosporine
- Hypercalcemia - hypercalcemia inhibits magnesium reabsorption
- Hyperaldosteronism
- Acute tubular necrosis
- Chronic metabolic acidosis
- Cystic fibrosis - possibly through decreased intestinal absorption and/or renal wasting
- Inherited tubular disorders (Gitelman syndrome, Bartter syndrome)
- Hypomagnesemia with secondary hypocalcemia - rare genetic disorder that causes nonfunctional magnesium channels in the intestine and kidneys
- Other rare genetic disorders
- Shift from extracellular to intracellular space
- Hungry bone syndrome - increased uptake by bones after correction of hyperparathyroidism
- Refeeding syndrome - refeeding syndrome occurs when a person who has been starved for a period is fed. The sudden increase in insulin and glucose stimulates cellular metabolism, which drives magnesium uptake by cells.
- Treatment of diabetic ketoacidosis - similar to refeeding syndrome, the sudden increase in insulin stimulates cellular metabolism, driving magnesium uptake by cells
- Hyperadrenergic states (e.g. alcohol withdrawal, post-surgery) - hyperadrenergic states can stimulate cellular magnesium uptake
- Acute pancreatitis - possibly from saponification of magnesium in necrotic fat, similar to what occurs with calcium [43,44]
- Hypermagnesemia
- Hypermagnesemia is defined as a magnesium level > 2.5 mg/dl. Hypermagnesemia is uncommon because the kidneys readily excrete excess magnesium. Causes of hypermagnesemia can be divided into three categories: (1) increased intake/absorption, (2) decreased renal elimination, (3) shift from intracellular to extracellular space.
- Increased intake/absorption
- Supplements, antacids, and laxatives that contain magnesium oxide, magnesium hydroxide, or magnesium citrate
- Eclampsia treatment and prevention in pregnant women - magnesium infusions with target serum levels of 4.2 - 8.4 mg/dl are used to prevent seizures
- Newborns of mothers treated with magnesium
- Gastrointestinal disorders (e.g. gastritis, colitis) - may increase absorption
- Decreased GI motility from anticholinergics or opiates - may increase absorption
- Decreased renal elimination
- Chronic kidney disease
- Lithium therapy - lithium inhibits magnesium excretion
- Milk-alkali syndrome
- Hypothyroidism
- Adrenal insufficiency
- Hyperparathyroidism - secondary to calcium-induced magnesium absorption in the renal tubule
- Familial hypocalciuric hypercalcemia - rare disorder that can sometimes cause hypermagnesemia (see familial hypocalciuric hypercalcemia above)
- Shift from intracellular to extracellular space
- Tissue breakdown (e.g. shock, burns)
- Tumor lysis syndrome
- Massive hemolysis
- Rhabdomyolysis
- Metabolic acidosis [45,46]
- Symptoms / ECG
- Symptoms
- Magnesium stabilizes neuronal axons and abnormal levels can affect nerve conduction
- Significant hypomagnesemia can increase neuron excitability, causing tremors, hyperreflexia, fasciculations, muscle cramps, tetany, and in extreme cases, seizures
- Mild hypermagnesemia (< 7 mg/dl) is typically asymptomatic, while levels above 7 mg/dl can suppress nerve conduction, causing loss of deep tendon reflexes, weakness, hypotension, confusion, ileus, and in extreme cases, paralysis.
- Magnesium also plays a role in myocardial electrical activity, and abnormal levels can cause the ECG changes described below. [43,44,45,46]
- ECG changes (hypomagnesemia)
- Widened QRS complex
- Peaked T waves
- Prolonged PR interval
- Premature atrial and ventricular beats
- Atrial fibrillation
- Ventricular arrhythmias including Torsades de Pointes [43,44]
- ECG changes (hypermagnesemia)
- Prolonged PR interval
- Bradycardia
- Atrioventricular block [45,46]
- Evaluation / Diagnosis
- The etiology of abnormal magnesium levels is typically evident from the patient history and other laboratories. In rare cases, a 24-hour urine for magnesium may be necessary to evaluate for abnormal renal magnesium handling. The fractional excretion of magnesium can also be determined from a spot urine using the following formula:
- Fractional excretion of magnesium (FEMg) = (UMg x PCr X 100) / (PMg X 0.7 X UCr)
- Where UMg is urine magnesium, PCr is plasma creatinine, PMg is plasma magnesium, and UCr is urine creatinine. PMg is multiplied by 0.7 since about 70% of magnesium is freely filtered in the glomerulus.
- In hypomagnesemia, a 24-hour urine magnesium of > 20 mg or a FEMg > 2% indicates renal magnesium loss and suggests abnormal renal wasting and/or drug effects (e.g. diuretics, aminoglycosides, cisplatin). A 24-hour urine magnesium < 20 mg or a FEMg < 2% indicates renal magnesium retention and suggests extrarenal losses. [43,44,45,46]
- Treatment
- Hypomagnesemia
- Hospitalized patients with severe (< 1.2 mg/dl) symptomatic hypomagnesemia should be treated with IV magnesium. Most patients with hypomagnesemia are asymptomatic and can be treated with oral supplements or dietary magnesium. General points about magnesium replacement are provided below. [43,44]
- Diuretic-induced hypomagnesemia - diuretic-induced hypomagnesemia can be treated with dietary magnesium, magnesium supplements, and/or the addition of an ENaC inhibitor (amiloride or triamterene), which promotes magnesium retention in the renal collecting duct (see magnesium retention with ENaC inhibitors for more). [44]
- Dietary magnesium - increased dietary magnesium intake can help to replenish stores. The recommended daily allowance in adults is around 420 mg in males and 320 mg in females. See foods sources of magnesium for a list of high-magnesium foods. The USDA food composition database also provides the magnesium content of thousands of foods. [44,48,49]
- Magnesium supplements - many over-the-counter magnesium supplements are available, although quality guidance on dosing and choosing a product is lacking. Magnesium is incompletely absorbed, and studies have found that the amount of bioavailable magnesium varies widely between formulations. The National Institute of Health states that the upper limit of tolerability for magnesium supplementation in adults is 350 mg of elemental magnesium per day; higher amounts will likely cause diarrhea and intolerable gastrointestinal side effects. However, most supplements provide low levels of bioavailable magnesium, so doses above this level are often used. Properties of available supplements are provided in the table below. Supplement dosing and length of therapy will depend upon the degree of deficiency and whether the underlying etiology has been addressed. Patients with significant kidney disease (GFR < 30 ml/min) are at increased risk of hypermagnesemia and should be started at half the typical dose and followed closely. Only a handful of studies have measured the effects of magnesium supplements on magnesium levels, and those studies are included in the table below. [43,44,47,48,49]
| Magnesium supplements (all supplements are available over the counter in various doses and formulations) |
||
|---|---|---|
| Magnesium supplement | Percent elemental magnesium | Comments |
| Magnesium oxide | 60% |
|
| Magnesium carbonate | 45% |
|
| Magnesium hydroxide | 42% |
|
| Magnesium citrate | 16% |
|
| Magnesium lactate | 12% |
|
| Magnesium chloride | 12% |
|
| Magnesium aspartate | 10% |
|
| Magnesium sulfate | 10% |
|
| Magnesium gluconate | 5% |
|
- Hypermagnesemia
- Symptomatic severe hypermagnesemia (> 12 mg/dl) should be treated with intravenous calcium because it blocks the neuromuscular and cardiovascular effects of magnesium. Normal saline infusions and loop diuretics can enhance magnesium excretion. In severe cases, hemodialysis may be necessary. [44,45]
BIBLIOGRAPHY
- 1 - PMID 9700180 - Hypokalemia, NEJM (1998)
- 2 - Chan, L. (2017). "Chapter 12 Electrolytes, Other Minerals, and Trace Elements". In Basic Skills in Interpreting Laboratory Data. Bethesda MD, USA: American Society of Health-System Pharmacists. Retrieved Oct 21, 2020, see ASHP link
- 3 - Guyton, Arthur C. Guyton And Hall Textbook Of Medical Physiology. Philadelphia, PA : Saunders/Elsevier, 13 ed. (2016)
- 4 - Rhoades, David R. Bell. Medical Physiology : Principles for Clinical Medicine. Philadelphia:Wolters Kluwer Health/Lippincott Williams & Wilkins, 5th ed. (2018)
- 5 - PMID 32966726 Case 30-2020: A 54-Year-Old Man with Sudden Cardiac Arrest, NEJM (2020)
- 6 - Medscape, Hypokalemia
- 7 - PMID 26371733 - Potassium Disorders: Hypokalemia and Hyperkalemia, American Family Physician, (2015)
- 8 - PMID 10979053 - New Guidelines for Potassium Replacement in Clinical Practice, Arch, Intern Med (2000)
- 9 - PMID 23817179 - Spurious electrolyte disorders: a diagnostic challenge for clinicians, Am J Nephrol (2013)
- 10 - PMID 18426576 - Practical aspects in the management of hypokalemic periodic paralysis, J Transl Med (2008)
- 11 - Clinical Pathology Laboratories test directory
- 12 - PMID 15295051 - Managing Hyperkalemia Caused by Inhibitors of the Renin–Angiotensin–Aldosterone System, NEJM (2004)
- 13 - Medscape, Hyperkalemia
- 14 - PMID 26880451 - Treatment of hyperkalemia: something old, something new, Kidney Int (2016)
- 15 - PMID 31119681 - Management of hyperkalemia in patients with kidney disease: a position paper endorsed by the Italian Society of Nephrology, J Nephrol (2019)
- 16 - Manufacturer's package insert for listed drug
- 17 - PMID 25830425 - Molecular Physiology of Water Balance, NEJM (2015)
- 18 - PMID 25551526 - Disorders of Plasma Sodium — Causes, Consequences, and Correction, NEJM (2015)
- 19 - PMID 29262111 - Rondon H, Badireddy M. Hyponatremia. 2020 Nov 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; (2020)
- 20 - Hyponatremia, Medscape
- 21 - PMID 26109207 - Mild Chronic Hyponatremia in the Ambulatory Setting: Significance and Management, Clin J Am Soc Nephrol (2015)
- 22 - PMID 10824078 - Hyponatremia, NEJM (2000)
- 23 - Pharmaceutical Calculations, 9th ed. Stoklosa, Ansel (1991)
- 24 - PMID 10816188 - Hypernatremia, NEJM (2000)
- 25 - PMID 28722989 - Sonani B, Naganathan S, Al-Dhahir MA. Hypernatremia. [Updated 2020 Aug 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-.
- 26 - Hypernatremia, Medscape
- 27 - Diabetes insipidus, Medscape
- 28 - Hypercalcemia, Medscape
- 29 - PMID 26675713 - Hypercalcemia of malignancy and new treatment options, Ther Clin Risk Manag (2015)
- 30 - Hyperparathyroidism, Medscape
- 31 - PMID 29083672 - Afzal M, Kathuria P. Familial Hypocalciuric Hypercalcemia. [Updated 2020 Jul 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459190/
- 32 - PMID 9690425 - Calcium, Lancet (1998)
- 33 - PMID 28613465 - Sadiq NM, Naganathan S, Badireddy M. Hypercalcemia. 2020 Sep 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–. PMID: 28613465.
- 34 - PMID 28613662 - Goyal A, Singh S. Hypocalcemia. 2020 Jun 22. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–.
- 35 - Hypocalcemia, Medscape
- 36 - PMID 30675420 - KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD), Kidney Int Suppl (2017)
- 37 - PMID 20830820 - Oral phosphate binders in patients with kidney failure, NEJM (2010)
- 38 - PMID 20610248 - Postoperative calcium requirements in 6,000 patients undergoing outpatient parathyroidectomy: easily avoiding symptomatic hypocalcemia, J Am Coll Surg (2011)
- 39 - PMID 31869067 - Goyal R, Jialal I. Hyperphosphatemia. 2020 Nov 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–.
- 40 - Hyperphosphatemia, Medscape
- 41 - Hypophosphatemia, Medscape
- 42 - PMID 29630224 - Sharma S, Hashmi MF, Castro D. Hypophosphatemia. 2020 Dec 1. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–.
- 43 - Hypomagnesemia, Medscape
- 44 - PMID 29763179 - Gragossian A, Bashir K, Friede R. Hypomagnesemia. 2020 Sep 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–.
- 45 - Hypermagnesemia, Medscape
- 46 - PMID 31747218 - Cascella M, Vaqar S. Hypermagnesemia. 2020 Dec 2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–.
- 47 - PMID 30987399 - Magnesium: The Forgotten Electrolyte-A Review on Hypomagnesemia, Med Sci (Basel) (2019)
- 48 - PMID 19621856 - Therapeutic uses of magnesium, Am Fam Physician (2009)
- 49 - Magnesium: Fact Sheet for Health Professionals, NIH
- 50 - Manufacturer's package insert
- 51 - PMID 35526553 - Pre-eclampsia with paradoxical polyuria: diabetes insipidus in pregnancy, Lancet (2022)
- 52 - Gubbi S, Hannah-Shmouni F, Koch CA, et al. Diagnostic Testing for Diabetes Insipidus. [Updated 2022 Nov 28]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537591/
- 53 - PMID 37966286 - Arginine or Hypertonic Saline-Stimulated Copeptin to Diagnose AVP Deficiency, NEJM (2023)
- 54 - PMID 34008000 - Performance of the Aldosterone to Renin Ratio as a Screening Test for Primary Aldosteronism, J Clin Endocrinol Metab, (2021)
- 55 - PMID 36413507 - Intra-individual Variability of Serum Aldosterone and Implications for Primary Aldosteronism Screening, J Clin Endocrinol Metab, (2023)
