Acronyms and definitions
- AACE - American Association of Clinical Endocrinology
- ATA - American Thyroid Association
- BMJ - British Medical Journal
- ETA - European Thyroid Association
- Levothyroxine (L-thyroxine) - pharmaceutical form of T4. Levothyroxine is identical to thyroxine secreted by the thyroid gland. [5]
- Liothyronine (L-triiodothyronine) - pharmaceutical form of T3
- NHANES III study - cross-sectional study of the U.S. population that measured thyroid-related labs on a random sample of 17,353 people ≥ 12 years old
- PPT - Postpartum thyroiditis
- rT3 - reverse T3
- Subclinical hypothyroidism - elevated TSH with a normal free T4 level
- TBG - Thyroxine-binding globulin
- Tg - Thyroglobulin
- TgAb - Thyroglobulin antibody
- TPOAb - Thyroid peroxidase antibodies
- USPSTF - United States Preventive Services Task Force
PHYSIOLOGY
- Thyroid hormone effects
- Thyroid hormone stimulates cellular gene transcription that leads to the production of proteins involved in a number of vital processes. Thyroid hormone has the following physiologic effects:
- Increase in cellular metabolic activity and maintenance of the normal basal metabolic rate
- Stimulation of heat production in tissues
- Release of growth hormone from the pituitary, which is necessary for normal development
- Growth and maturation of the brain in utero and during the neonatal period
- Stimulation of carbohydrate, protein, and fat metabolism
- Reduction in circulating cholesterol, phospholipids, and triglycerides [30,31]
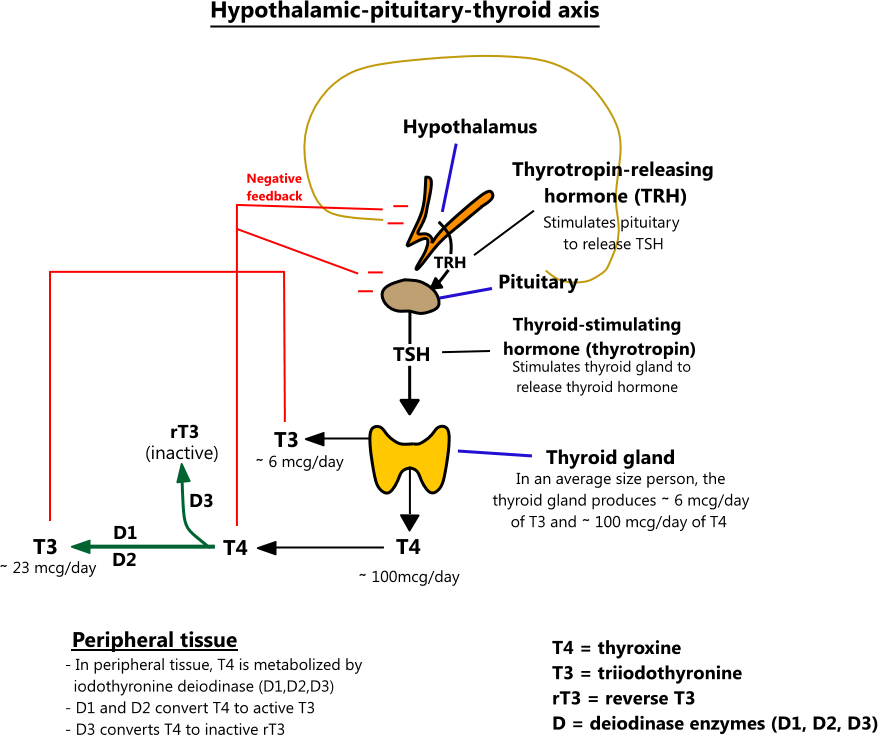
- Hypothalamic-pituitary-thyroid axis
- Thyrotropin-releasing hormone (TRH) from the hypothalamus causes the pituitary to secrete TSH. TSH stimulates the thyroid gland to secrete T4 and T3, which exert negative feedback on the hypothalamus and pituitary.
- In an average size person, the thyroid gland produces ∼ 100 mcg of T4 and ∼ 6 mcg of T3 per day. In the periphery, T4 is metabolized by iodothyronine deiodinase, an enzyme that has 3 subtypes - D1, D2, and D3. D1 converts T4 to T3 and is located primarily in the liver, kidneys, and thyroid gland. D2 works locally in tissues (brain, pituitary, muscle, placenta) to maintain normal intracellular T3 levels when plasma T3 and T4 levels fluctuate. D3 converts T4 to reverse T3 (rT3), an inactive form of thyroid hormone. D3 activity is increased in states where the body is trying to conserve energy (e.g. fasting, trauma, severe illness). [2,4,30,31]
PREVALENCE OF HYPOTHYROIDISM
- Overview
- The prevalence of hypothyroidism varies by age, ethnicity, and sex. Women are affected more than men, and the incidence increases as people grow older. In the Framingham Heart Study, 5.9% of women over 60 had a TSH value > 10 mU/L compared to 2.3% of men. Of these patients, 39% had low T4 values. [7]
- Data from the the NHANES III study, a cross-sectional study of the general U.S. population, is presented below
| Prevalence of hypothyroidism in U.S. population ≥ 12 years old | |||||
|---|---|---|---|---|---|
| Total | White | Black | Mexican-American | Remaining races | |
| Subclinical hypothyroidism (% of population) |
4.3% | 4.8% | 1.6% | 3.9% | 4.0% |
| Clinical hypothyroidism (% of population) |
0.3% | 0.4% | 0.1% | 0.2% | 0.2% |
RISK FACTORS FOR HYPOTHYROIDISM
- Primary hypothyroidism
- Primary hypothyroidism occurs when the thyroid gland fails to produce adequate amounts of thyroid hormone. It is by far the most common cause of hypothyroidism.
| RISK FACTORS FOR PRIMARY HYPOTHYROIDISM | |
|---|---|
| Risk factor | Notes |
| Iodine deficiency |
|
| Female sex |
|
| Advancing age |
|
| Ethnicity |
|
| Family history of autoimmune thyroid disease | |
| Thyroid peroxidase antibodies (TPOAb) |
|
| Pregnancy |
|
| History of thyroid disease/treatment |
|
| Medications | |
| Presence of other autoimmune disease |
|
| Genetic disorders |
|
- Secondary hypothyroidism
- Secondary hypothyroidism, also called central hypothyroidism, occurs when the hypothalamic-pituitary axis fails to stimulate the thyroid gland to produce thyroid hormone (see hypothalamic-pituitary-thyroid axis above). Secondary hypothyroidism is much less common than primary hypothyroidism.
| RISK FACTORS FOR SECONDARY HYPOTHYROIDISM | |
|---|---|
| Cause | Notes |
| Pituitary or hypothalamic tumors |
|
| Infiltrative inflammatory diseases |
|
| Medications | |
| Iatrogenic |
|
| Hemorrhagic necrosis |
|
SYMPTOMS OF HYPOTHYROIDISM
- Overview
- Symptoms of hypothyroidism are often subtle, have low sensitivity, and are highly prevalent in patients without thyroid disease
- Symptoms of severe, chronic hypothyroidism are more profound but much less common
- Symptoms of hypothyroidism
- Hoarse voice
- Deep voice
- Constipation
- Dry skin
- Cold intolerance - discomfort in a cool environment
- Fatigue
- Puffy eyes
- Muscle cramps and weakness
- Irregular menses - typically polymenorrhea
- Decreased cognitive function - poor memory, slow thinking [8]
- Symptoms of severe hypothyroidism
- Hyponatremia (low sodium) caused by impaired free water excretion and SIADH
- Myxedema - nonpitting edema with mucin deposits of the skin
- Hypothermia
- Respiratory failure
- Ileus
- Severe cognitive decline - delirium, dementia, seizure, coma
- Adrenal insufficiency
- Pituitary hyperplasia
- Coagulopathy - von Willebrand syndrome, decreased Factor V, VII, VIII, IX, X
- Cretinism - syndrome of mental retardation, deafness, short stature, and facial deformities seen in congenital hypothyroidism
- Sleep apnea
- Carpal tunnel syndrome [1,9]
SCREENING FOR HYPOTHYROIDISM
| Screening recommendations for asymptomatic hypothyroidism | |
|---|---|
| Organizations | Screening recommendation |
|
|
|
|
|
|
AUTOIMMUNE THYROIDITIS (HASHIMOTO'S THYROIDITIS)
- Overview
- In iodine-sufficient areas, the most common cause of hypothyroidism is chronic autoimmune thyroiditis. Autoimmune thyroiditis was first described by Hakaru Hashimoto in 1912 and is therefore sometimes referred to as Hashimoto's thyroiditis.
- The pathology of autoimmune thyroiditis is characterized by lymphocytic infiltration of the thyroid gland and the development of thyroid autoantibodies. Thyroid peroxidase antibodies are present in 90% of cases, and thyroglobulin antibodies in 20 - 50%.
- The cause of autoimmunity in thyroiditis is not entirely understood. Proposed theories include a precipitating viral infection of the thyroid gland, genetic susceptibility (e.g. HLA-DR3, HLA-DR4, HLA-DR5), and environmental factors (e.g. smoking).
- Over time, thyroiditis leads to the depletion of thyroid hormone, and hypothyroidism develops. Depending on the stage, autoimmune thyroiditis may present with goiter, a normal size thyroid gland, or an atrophied thyroid gland. [1,14]
- Risk factors for autoimmune thyroiditis include the following:
- Female sex
- Advancing age
- Family history of autoimmune thyroid disease
- Personal history of autoimmune disease
- Smoker [1,14]
THYROID LABS
- Thyroid-stimulating Hormone (TSH)
- TSH, also called thyrotropin, is secreted by the pituitary in response to TRH. TSH stimulates the thyroid gland to release T3 and T4. In return, rising T4 and T3 levels act on the pituitary gland to suppress TSH secretion. (see hypothalamic-pituitary-thyroid axis above). TSH levels are therefore inversely related to circulating T4 and T3 levels.
- TSH is the most common lab used to monitor therapy and screen for thyroid disease. TSH is very sensitive to changes in circulating free T4 levels, so much so that abnormal TSH levels tend to precede abnormal free T4 levels in thyroid disease.
- TSH levels tend to be lowest in the late afternoon and highest at bedtime. Levels can vary daily by as much as 50% from the average level.
- The normal range for a TSH level used by most labs is 0.45 - 4.5 mIU/L. Studies have shown that normal TSH ranges tend to increase with age and vary by ethnicity. Data from the NHANES III study is presented in the table below.
| TSH Values in Thyroid Disease-free Population 2.5th - 97.5th percentile TSH (mIU/L) values |
|||||||
|---|---|---|---|---|---|---|---|
| Age range | 20 - 29 (Median) |
30 - 39 (Median) |
40 - 49 (Median) |
50 - 59 (Median) |
60 - 69 (Median) |
70 - 79 (Median) |
≥ 80 (Median) |
| Black | 0.36 - 3.30 (1.10) |
0.33 - 3.24 (1.10) |
0.42 - 3.74 (1.30) |
0.44 - 3.99 (1.40) |
0.35 - 4.20 (1.58) |
0.39 - 5.20 (1.50) |
0.42 - 4.60 (1.50) |
| Mexican-American | 0.47 - 3.62 (1.33) |
0.40 - 3.75 (1.30) |
0.40 - 3.99 (1.49) |
0.55 - 4.85 (1.50) |
0.51 - 5.54 (1.80) |
0.59 - 7.12 (2.13) |
0.55 - 7.84 (1.91) |
| White | 0.46 - 3.60 (1.30) |
0.46 - 3.76 (1.37) |
0.57 - 3.95 (1.49) |
0.52 - 3.97 (1.58) |
0.56 - 4.31 (1.66) |
0.46 - 5.60 (1.80) |
0.41 - 6.56 (1.99) |
- Thyroxine (T4)
- Thyroxine, also referred to as T4, is the main form of thyroid hormone secreted by the thyroid gland (∼ 100 mcg/day in an average size person). In peripheral tissues (e.g. liver, kidney, brain, skeletal muscle), T4 is converted to T3, which is more potent than T4.
- Approximately 99.97% of T4 circulates bound to protein, primarily thyroxine-binding globulin, and to a lesser extent, transthyretin. The small unbound portion is called free T4, and it is the active form. Total T4 is the amount of free and bound T4.
- Changes in thyroxine-binding globulin levels can alter total T4 levels (see factors affecting TBG) so that they may not reflect true T4 activity. For this reason, free T4 is the preferred assay when assessing thyroid levels.
- High TSH levels with low free T4 levels are seen in primary hypothyroidism, and low or normal TSH levels with low free T4 levels are seen in secondary hypothyroidism.
- When monitoring thyroid replacement, levels should be drawn before the next dose (trough level) because free T4 levels will be transiently increased for up to 9 hours after dosing [1]
- Normal free T4 in adults: 0.82 - 1.77 ng/dl
- Normal total T4 in adults: 4.5 - 12 mcg/dl [17]
- Triiodothyronine (T3)
- Triiodothyronine, also referred to as T3, is the most active form of thyroid hormone. T3 is 3 - 4 times more potent than T4. Most T3 is formed in peripheral tissues (e.g. liver, kidney, brain, skeletal muscle) from the conversion of T4 to T3 (∼ 23 mcg/day), while only a small amount is secreted by the thyroid gland (∼ 6 mcg/day).
- Approximately 99.97% of T3 circulates bound to protein, primarily thyroxine-binding globulin, and to a lesser extent, transthyretin. The small unbound portion is called free T3, and it is the active form. Total T3 is the amount of free and bound T3.
- Changes in thyroxine-binding globulin levels can alter total T3 levels (see factors affecting TBG) so that they may not reflect true T3 activity. For this reason, free T3 is the preferred assay when assessing thyroid levels.
- Free T3 levels have limited utility in evaluating hypothyroidism because they are often normal in hypothyroidism secondary to increased peripheral conversion and thyroid hyperstimulation from TSH. T3 levels may also be spuriously low in the absence of thyroid disease, particularly in patients with multiple medical problems. [1,4]
- Normal free T3 range in adults: 2.0 - 4.4 pg/ml
- Normal total T3 in adults: 71 - 180 ng/dl [17]
- T3 resin uptake / Free thyroxine Index (T7)
- T3 resin uptake is also referred to as "free thyroxine index (T7)" and "thyroid hormone-binding ratio." Despite the name, T3 resin uptake does not measure T3. Instead, T3 resin uptake is an indirect measure of thyroxine-binding globulin capacity.
- T3 resin uptake and total T4 measurements can be used to estimate the free T4 index, which provides an estimate of free T4. Because free T4 levels can now be measured directly, the T3 resin uptake has fallen out of favor, but it is still sometimes measured in pregnancy because it provides a more accurate estimation of free T4 activity in pregnant women (see free T4 in pregnancy for more).
- The procedure for measuring the T3 resin uptake is detailed below
- Normal range for T3 resin uptake in adults: 24 - 39% [17]
- Procedure:
- T3 resin binder is mixed with patient's serum
- A small amount of I¹²⁵-T3 (radiolabeled T3) is added to the mixture
- I¹²⁵-T3 binds to binding sites on the patient's thyroxine-binding globulin (TBG) and the T3 resin binder
- The T3 resin binder is then removed from the mixture and the amount of I¹²⁵-T3 activity is measured
- If the patient has a low amount of T4 (hypothyroid), then more binding sites on the TBG will be available for the I¹²⁵-T3, and a smaller amount of I¹²⁵-T3 will be bound to the resin (lower activity)
- If the patient has a high amount of T4 (hyperthyroid), then few binding sites on the TBG will be available for the I¹²⁵-T3, and a larger amount of I¹²⁵-T3 will be bound to the resin (higher activity)
- Increases and decreases in TBG will also affect the T3 resin uptake. Increases in TBG will cause the resin uptake to be lower, and decreases in TBG will cause it to be higher. [19]
- Calculating the Free T4 (thyroxine) index (T7)
- The T3 resin uptake and the total T4 can be used to calculate the Free T4 index (sometimes called T7) with the formula below
- Normal free thyroxine index in adults: 1.4 - 3.8 [18]
- Free T4 Index = Total T4 in patient X [(T3 resin uptake in patient)/(T3 resin uptake in lab control)]
- Interpreting T3 resin uptake results
- Expected values for the T3 resin uptake and the total T4 are presented in the table below
| Hyperthyroidism | Hypothyroidism | High TBG | Low TBG | |
|---|---|---|---|---|
| Total T4 | High | Low | High | Low |
| T3 resin uptake | High | Low | Low | High |
- Thyroxine-binding globulin (TBG)
- The majority of T4 and T3 circulates in the blood bound to TBG. Only unbound (free) T4 and T3 are active. Changes in TBG levels can affect the amount of free T4 and free T3. Since free T4 levels can be measured directly, TBG levels have little value in diagnosing hypothyroidism and are mainly used to diagnose congenital TBG anomalies. Conditions that can affect TBG are listed below.
- Normal range for TBG in adults: 13 - 39 mcg/ml [16]
- Factors that increase TBG
- Inherited
- Pregnancy
- Neonatal state
- Estrogens
- Hepatitis
- Porphyria
- Clofibrate
- Heroin
- Methadone
- Mitotane
- 5-Fluorouracil
- SERMs (tamoxifen, raloxifene)
- Perphenazine
- Factors that decrease TBG
- Inherited
- Androgens
- Anabolic steroids
- Glucocorticoids
- Severe illness
- Liver failure
- Kidney disease
- Niacin
- L-Asparaginase
- Acromegaly
- Factors that alter T4 and T3 binding to TBG
- Salicylates (e.g. aspirin)
- Furosemide (Lasix®) at doses > 80 mg
- Free fatty acids
- Phenytoin
- Carbamazepine
- NSAIDs
- Heparin
- Thyroid peroxidase antibodies (TPOAbs)
- Thyroid peroxidase is an enzyme involved in the formation of thyroid hormone. Thyroid peroxidase antibodies, also called anti-microsomal antibodies, are autoimmune antibodies formed against the thyroid peroxidase enzyme.
- TPOAbs are a risk factor for the development of autoimmune thyroiditis. In patients with subclinical hypothyroidism and elevated TPOAb, 4.3% per year will develop overt hypothyroidism compared to 2.6% in patients without elevated TPOAbs.
- High serum TPOAbs are present in 90% of patients with Hashimoto's thyroiditis [1,14]
- TPOAbs also occur in patients without thyroid disease. In the NHANES III study, 14% of women without thyroid disease had TPOAbs (defined as TPOAb ≥ 0.5 U/ml), which were more prevalent in females and Whites and increased with age. Data from the NHANES III study is presented in the table below.
- Normal range for TPOAb in adults: < 9 IU/ml [18]
| Percent of Thyroid Disease-free Patients with Positive TPOAb | |||||||
|---|---|---|---|---|---|---|---|
| Age range | 20 - 29 | 30 - 39 | 40 - 49 | 50 - 59 | 60 - 69 | 70 - 79 | ≥ 80 |
| Female | 10.4 | 12.6 | 15.8 | 17.1 | 23 | 26.2 | 26.5 |
| Male | 5.5 | 8.4 | 10.6 | 10.1 | 10.2 | 12 | 10.6 |
- Thyroglobulin (Tg) and thyroglobulin antibody (TgAb)
- Thyroglobulin is the main storage form of T4 and T3, and it serves as a precursor molecule in their synthesis. Antibodies to thyroglobulin are found in some patients with autoimmune thyroiditis (e.g. Hashimoto disease, postpartum thyroiditis, neonatal hypothyroidism, Graves disease), but they are also very prevalent in people without thyroid disease (see table below). In contrast to TPOAbs, thyroglobulin antibodies have not been found to be a risk factor for hypothyroidism. [1,14]
- Thyroglobulin levels are primarily used to monitor for the recurrence of thyroid cancer in patients who have had their thyroid removed. Thyroglobulin is only produced by thyroid tissue, so its presence indicates that thyroid tissue remains. A thyroglobulin antibody titer is typically performed with a thyroglobulin level, because thyroglobulin antibodies can bind circulating thyroglobulin and interfere with its measurement. When antibodies are present, special testing must be performed in order to measure thyroglobulin levels accurately. Thyroglobulin antibodies can also serve as a surrogate tumor marker because their persistence indicates that thyroglobulin is present. [17]
- Normal range for TgAb in adults: < 4 IU/ml [18]
| Percent of Thyroid Disease-free Patients with Positive TgAb | |||||||
|---|---|---|---|---|---|---|---|
| Age range | 20 - 29 | 30 - 39 | 40 - 49 | 50 - 59 | 60 - 69 | 70 - 79 | ≥ 80 |
| Female | 8.5 | 13.6 | 16 | 16.4 | 19.6 | 20.6 | 25.2 |
| Male | 5 | 6.6 | 6.8 | 7.9 | 9.6 | 12.9 | 10.1 |
- Reverse T3 (rT3)
- Reverse T3 (rT3) is the biologically inactive form of T3. Most rT3 is produced in the periphery, and the thyroid secretes a small amount.
- Three different iodothyronine deiodinase enzymes metabolize T4 (abbreviated D1, D2, and D3). T3 is produced when D1 and D2 metabolize T4, and rT3 is produced when D3 metabolizes T4. (see hypothalamic-pituitary-thyroid axis above)
- During times of physiologic stress (e.g. myocardial infarction, severe illness, cancer), levels of rT3 rise. It is hypothesized that the shift from T3 to rT3 production is a response by the body to conserve energy.
- rT3 levels are not useful in most thyroid disorders. However, they can help distinguish central hypothyroidism (low rT3) from nonthyroidal illness (high rT3). [16,17,27]
- Biotin supplements
- Biotin is a B vitamin promoted to strengthen nails and hair and improve metabolism, among other things. Some thyroid assays, including TSH, free T3, free T4, and thyroglobulin, use biotin as a reagent. Biotin supplementation can increase plasma levels and interfere with testing; therefore, biotin supplements should be stopped at least two days before thyroid labs are drawn.
MEDICATIONS THAT CAN AFFECT THYROID HORMONE
- See drug interactions for a list of medications that can interfere with exogenous and endogenous thyroid hormones
OBESITY AND THYROID DISEASE
- A wide-held perception among patients and healthcare providers is that hypothyroidism and obesity are strongly linked. This leads to frequent screening of overweight patients for thyroid disease. However, studies have shown that hypothyroidism is no more common in the obese population than in the general population. Furthermore, patients with marked hypothyroidism often have suppressed appetites which offset the decrease in metabolic activity.
- TSH levels tend to rise with increasing BMI, but they have also been shown to fall and revert to normal in patients who experience profound weight loss (e.g. bariatric surgery). This observation appears to show that elevated TSH levels may be a marker of obesity as opposed to a cause.
- Providers should use caution when treating patients with subclinical hypothyroidism and obesity. Elevated TSH levels may actually reflect the obese state rather than a hypothyroid state. [1,23]
TREATMENT OF HYPOTHYROIDISM
- Overview
- Primary hypothyroidism (elevated TSH and low free T4) should be treated with levothyroxine replacement therapy. The goal of treatment is to achieve a TSH value in the normal range. Because symptoms of hypothyroidism have low sensitivity and are highly prevalent in patients with normal thyroid function, a treatment goal of symptom resolution is not recommended.
- Levothyroxine therapy (T4)
- Levothyroxine is the pharmaceutical form of T4 and is identical to thyroxine secreted by the thyroid gland
- All major professional associations (AACE, ATA, ETA) recommend levothyroxine monotherapy be used for thyroid replacement therapy. Combination products with levothyroxine and triiodothyronine are not recommended. (see combination therapy).
- Levothyroxine dosage depends on the patient's age, thyroid status, body weight, and TSH level. The table below gives general dosing recommendations from the AACE and ATA.
- Most patients should be treated to achieve a normal TSH value. Patients with a history of thyroid cancer may have lower goals.
| Levothyroxine dosing recommendations |
|---|
|
Little residual thyroid function or markedly elevated TSH
|
TSH ≤ 10 mIU/L
|
|
Dosing based on TSH level
|
Elderly patients
|
Patients with coronary artery disease
|
| Pregnancy |
Food
|
Intravenous levothyroxine
|
- Monitoring therapy
- Recheck TSH levels 4 - 8 weeks after the following: therapy initiation, dose adjustments, change in levothyroxine product
- Adjust dose in increments of 12.5 - 25 mcg per day
- For small dose adjustments, TSH levels may take 8 weeks or longer to stabilize
- Symptoms of hypothyroidism (e.g. dry skin) may take 3 - 6 months to resolve after TSH levels have normalized
- Once TSH levels have normalized, recheck TSH at 6 months and then yearly
- Switching levothyroxine products
- Many different levothyroxine products are available. Some products are rated bioequivalent to each other, while others are not (see thyroid preparations).
- Over the years, patients have complained that switching products affects the efficacy of their therapy. Case reports of TSH variations after brand changes have been reported, but studies evaluating this phenomenon have been mixed.
- In general, patients should stay with the same product if possible. If a brand change occurs, significant changes in TSH and efficacy are unlikely, but checking a TSH after 4 - 8 weeks is reasonable. [20,29]
- Combination therapy (T3 + T4) versus levothyroxine (T4) therapy
- Some patients do not perceive symptom relief on levothyroxine monotherapy despite normalization of TSH levels. When this occurs, some providers will change patients to combination therapy containing T4 (levothyroxine) and T3 (liothyronine). Combination therapy may be achieved by prescribing a T3 product (e.g., Cytomel®) with levothyroxine or a single product containing T4 and T3 (e.g., Armour® thyroid).
- All major professional associations recommend levothyroxine monotherapy for hypothyroidism. Reasons cited for this position include the following:
- Combination products (e.g. Armour® thyroid) have a T4 to T3 ratio of 4:1. Physiologic ratios secreted by the thyroid gland are around 14:1.
- Combination therapy leads to supraphysiologic levels of T3, which may lead to symptoms of thyrotoxicosis
- T3 has a shorter half-life than T4, which can lead to fluctuations in T3 levels, with a peak occurring shortly after dosing
- There is substantially more data from trials on the use of levothyroxine compared to combination therapy
- In general, randomized controlled trials comparing combination therapy to levothyroxine monotherapy have found no difference in symptom relief, quality of life, body weight, and lipid parameters [1,4,20,24,32]
- Combination therapy dosing
- Guidelines for combination therapy from the ETA are presented below. The ETA states that levothyroxine monotherapy is preferred but recognizes that some patients and providers may favor combination therapy.
- Product selection
- Separate T4 and T3 products should be used for combination therapy so that physiologic ratios of T4 and T3 can be achieved
- Cytomel® is a common T3 product, and Synthroid® and Levoxyl® are standard T4 products. See thyroid preparations for more
- Products containing both T4 and T3 (e.g. Armour® thyroid) are not recommended because their ratios of T4 to T3 are not physiologic. For example, Armour thyroid contains 38 mcg of levothyroxine and 9 mcg of liothyronine per 60 mg giving it a T4 to T3 ratio of 4.2:1, while the physiologic ratio of T4 to T3 is 14:1. [4]
- Calculating dosage
- The ETA lists 4 similar methods for calculating T4 and T3 doses. One method is detailed below.
- The goal of therapy is to achieve a T4 to T3 ratio between 13:1 and 20:1 while taking into account their pharmacodynamic equivalence (bioavailability, etc.). If possible, the liothyronine dose should be given in two divided doses - one before breakfast and the largest before sleeping. Levothyroxine should be given once daily in the morning.
- Step 1 - Take the levothyroxine dose that has normalized TSH (designated "StartT4")
- Step 2 - T3 dose = StartT4 / 17
- Step 3 - New T4 dose = StartT4 - 3(T3 dose)
- Example:
- Step 1 - Patient is on 100 mcg of levothyroxine and has normal TSH. StartT4 = 100 mcg
- Step 2 - T3 dose = 100 mcg / 17 = 5.88 mcg
- Step 3 - New T4 dose = 100 mcg - 3(5.88 mcg) = 82.35 mcg
- Step 4 - After rounding to available dosage forms, the patient could be given liothyronine 6.25 mcg (1 and 1/4 of liothyronine 5 mcg) and levothyroxine 88 mcg [4]
- Monitoring therapy
- Thyroid tests (TSH, free T4, free T3) should be checked 6 - 8 weeks after starting therapy and with dosage changes
- Blood should be drawn before the morning dose
- If a dose adjustment is necessary, it is recommended that only one component be changed, preferably the T3 [4]
- Risks of overtreatment
- The main risks of thyroid hormone overtreatment are atrial fibrillation and osteoporosis. Postmenopausal women and patients 65 years and older may be at greater risk. A cohort study found that higher free T4 levels were associated with a greater risk of sudden cardiac death in patients ≥ 45 years old. [PMID 27601558]
- In general, TSH levels below 0.1 mIU/L should be avoided [23]
- Some patients with a history of thyroid cancer are given supraphysiologic doses of levothyroxine to suppress TSH levels. The risks and benefits of this practice are controversial and not discussed here.
SUBCLINICAL HYPOTHYROIDISM
- Subclinical hypothyroidism
- Subclinical hypothyroidism is a condition where the TSH is elevated, but the free T4 is normal. The proper treatment of affected patients is controversial. In general, there is no clear evidence from studies that thyroid replacement therapy improves symptoms or outcomes. A placebo-controlled trial published in 2017 found no benefit in treating subclinical hypothyroidism in elderly patients. [PMID 28402245] A subsequent meta-analysis of randomized trials also found no effect. [PMID 30285179] Another study published in 2020 found that treating subclinical hypothyroidism in patients with myocardial infarction did not improve ejection fraction at 52 weeks compared to placebo. [PMID 32692386]
- Subclinical hypothyroidism resolves spontaneously in many patients. In one study (N=459), adults ≥ 65 years with subclinical hypothyroidism were followed for four years. At two years, 46% of patients with a baseline TSH of 4.5 - 6.9 reverted to a normal TSH. For patients with a baseline TSH of 7 - 9.9, 10% returned to normal, and for those with a baseline ≥ 10, 7% returned to normal. [PMID 22438233] A similar study (N=2335) found that TSH levels normalized in 60.8% of patients after 1 year. [PMID 37862463]
- The BMJ and ETA have published recommendations for treating subclinical hypothyroidism. The BMJ guidelines published in 2019 recommend against treatment in most patients. The ETA guidelines published in 2013 give conditional guidance based on the patient's age and TSH value. Both guidelines are provided below.
- 2019 BMJ recommendations on treating subclinical hypothyroidism
- Do not treat patients with elevated TSH (up to 20 mIU/L) and a normal free T4 who are asymptomatic or report nonspecific symptoms (e.g. fatigue, constipation, poor memory)
- Recommendation may not apply to young adults (≤ 30 years) and/or patients with severe symptoms
- Recommendation does not apply to women who are trying to become pregnant and patients with TSH > 20 mIU/L [28]
| 2013 ETA recommendations for treating subclinical hypothyroidism | ||
|---|---|---|
| Age | TSH value | Recommendation |
| ≤ 70 years | < 10 |
|
| ≤ 70 years | ≥ 10 |
|
| > 70 years | < 10 |
|
| > 70 years | ≥ 10 |
|
PREGNANCY AND THYROID DISEASE
- Physiology
- Pregnancy has significant effects on the thyroid gland. Human chorionic gonadotropin (HCG) released from the placenta has TSH-like properties, and it exhibits negative feedback on the hypothalamus and pituitary, leading to a decrease in TSH levels. Pregnancy-induced increases in estrogen raise thyroxine-binding globulin levels by up to 50%, further complicating the interpretation of thyroid labs.
- Hypothyroidism is important to address during pregnancy because it can increase the risk of premature birth, gestational hypertension, low birth weight, miscarriage, neonatal respiratory distress, and impaired fetal neurocognitive development [21]
- Screening
- Several large randomized controlled trials have looked at universal hypothyroidism screening in pregnant women and generally found no benefit [21]
- Screening recommendations from different organizations are presented in the table below
| Screening for thyroid disease in asymptomatic pregnant females | |
|---|---|
| Organization | Screening recommendation |
| American Endocrine Society | No consensus agreement on whether or not to screen |
| American Thyroid Assoc | ✝See below |
| American College of Obstetricians and Gynecologists | Does not recommend universal screening |
| European Thyroid Assoc | Does not recommend routine, universal screening |
- ✝The ATA recommends screening the following patients
- A history of hypothyroidism/hyperthyroidism or current symptoms/signs of thyroid dysfunction
- Known thyroid antibody positivity or presence of a goiter
- History of head or neck radiation or prior thyroid surgery
- Age > 30 years
- Type 1 diabetes or other autoimmune disorders
- History of pregnancy loss, preterm delivery, or infertility
- Multiple prior pregnancies (≥ 2)
- Family history of autoimmune thyroid disease or thyroid dysfunction
- Morbid obesity (BMI ≥ 40)
- Use of amiodarone or lithium, or recent administration of iodinated radiologic contrast
- Residing in an area of known moderate to severe iodine insufficiency
- Thyroid labs in pregnancy
- Thyroid-stimulating hormone (TSH)
- Human Chorionic Gonadotropin (HCG) released from the placenta has TSH-like properties, and it exhibits negative feedback on the hypothalamus and pituitary, leading to a decrease in TSH levels. Because of this, the upper and lower limits of a normal TSH are decreased in pregnancy; TSH levels are lowest during the first trimester when HCG levels are highest, and they gradually increase throughout pregnancy as HCG declines.
- Many labs provide trimester-specific TSH ranges for pregnant women. The ATA recommends using these values if they are available. When trimester-specific values are unavailable, the 2017 ATA guidelines recommend that during the first trimester, the TSH lower limit of normal be reduced by 0.4 mU/L, while the upper limit of normal is reduced by 0.5 mU/L. For most assays, this will represent a TSH upper limit of normal of 4.0 mU/L. These limits should be applied around weeks 7 - 12 of pregnancy, with a gradual return to nonpregnant values during the second and third trimesters. [26]
- Free T4 levels
- Physiologic changes associated with pregnancy can affect free T4 measurements. Pregnant women have lower albumin levels and higher levels of TBG and fatty acids. These changes can invalidate free T4 immunoassays.
- The best method for measuring free T4 levels in pregnancy is controversial. The ATA states that "the accuracy of serum free T4 measurement by the indirect analog immunoassays is influenced by pregnancy and also varies significantly by manufacturer. If measured in pregnant women, assay method-specific and trimester-specific pregnancy reference ranges should be applied." They go on to say that total T4 measurements with pregnancy-adjusted reference ranges are reliable during the last part of pregnancy, and the free thyroxine index can also be used to estimate free T4 concentrations. [26]
- The Endocrine Society states that nonpregnant total T4 values can be multiplied by 1.5 to estimate normal pregnant values in the second and third trimesters. It also states that the free thyroxine index appears to be valid in pregnancy. [21]
- Thyroid antibodies (TPOAb and TgAb)
- 10 - 20% of pregnant women are positive for thyroid antibodies. Thyroid antibodies are associated with an increased risk of hypothyroidism, miscarriage, and postpartum thyroiditis.
- Currently, the ATA and the Endocrine Society do not recommend screening euthyroid women for thyroid antibodies [20,21]
- Pre-existing hypothyroidism treatment (ATA recommendations)
- Prior to pregnancy
- Women with hypothyroidism who are planning pregnancy should have their levothyroxine doses adjusted to achieve a TSH value < 2.5 mIU/L
- During pregnancy
- Treat to achieve trimester-specific TSH values (see TSH in pregnancy above)
- The majority of newly pregnant women will require increased doses of levothyroxine during pregnancy
- Two acceptable methods for the initial increase in levothyroxine dose after pregnancy occurs are:
- Increase daily levothyroxine dose by 20 - 30%
- Increase current levothyroxine dose from once daily to 9 doses per week
- Monitor TSH levels every 4 weeks during first half of pregnancy
- Check TSH at least once between 28 and 32 weeks
- After delivery, reduce levothyroxine dose to preconception levels and check TSH 6 weeks postpartum
- T3 products (ex. Armour thyroid) are not recommended [21,26]
- Subclinical hypothyroidism treatment (ATA recommendations)
- Definition
- Subclinical hypothyroidism in pregnancy is defined as a TSH of 2.5 - 10 mIU/L with a normal free T4
- Whom to treat
- Women with subclinical hypothyroidism who are TPOAb positive should be treated
- Women with subclinical hypothyroidism who are TPOAb negative and have a TSH > 10 mU/L should be treated
- Treatment guidelines are the same as for women with overt hypothyroidism (see above)
- Women with subclinical hypothyroidism who are not initially treated should be monitored with a serum TSH and Free T4 approximately every 4 weeks until 16 – 20 weeks gestation and at least once between 26 and 32 weeks gestation [21,26]
- Studies
- A study published in 2017 found that treating subclinical hypothyroidism during pregnancy did not affect the cognitive function of offspring [PMID 28249134]
- A study published in 2020 found that treating subclinical hypothyroidism during pregnancy had no effect on maternal depression [PMID 32168208]
- Positive thyroid antibodies and normal TSH/T4 (ATA recommendations)
- Monitoring
- Check TSH at the time of pregnancy confirmation and every 4 weeks during the first half of pregnancy [21,26]
- Studies
- A study published in 2019 found no effect of thyroid supplementation on live birth rates among women with normal thyroid function and positive thyroid peroxidase antibodies. [PMID 30907987]
- Postpartum thyroiditis (PPT)
- Postpartum thyroiditis is an autoimmune inflammatory syndrome marked by lymphocytic infiltration of the thyroid gland in the first few months after delivery. It occurs in up to 10% of women in the U.S, and patients with thyroid antibodies, other autoimmune diseases (e.g. type 1 diabetes), and a family history of thyroid disorders are at greater risk.
- PPT typically causes hyperthyroidism that begins within 1 - 6 months following delivery and lasts for 1 - 2 months. A hypothyroid phase may then follow that lasts 4 - 6 months.
- 80% of women will return to normal thyroid function within a year. Treatment is usually symptomatic, with as-needed beta blockers for hyperthyroid symptoms. If a prolonged hypothyroid phase occurs, temporary levothyroxine may be required.
- PPT can be distinguished from Graves' disease by several methods. TSH receptor antibodies are typically present in Graves' disease and absent in PPT. A radioiodine uptake test is usually elevated in Graves' disease and low in PPT. Radioiodine is passed in breast milk, and breastfeeding women should pump and discard breast milk for at least 2 days following the study.
- Recurrent PPT is seen in 70% of subsequent pregnancies, and 50% of affected women will go on to develop hypothyroidism within 7 years [14,21]
BIBLIOGRAPHY
- 1 - PMID - 22954017 - AACE 2012 Hypothyroid guidelines
- 2 - PMID - 19915227 - Thyroid axis paper
- 3 - Medical Physiology, Rhoades and Tanner, 1st ed., 1995
- 4 - PMID - 24782999 - ETA statement on T4 and T3
- 5 - Synthroid PI
- 6 - PMID 11836274 - NHANES III study
- 7 - PMID 4026469 - Framingham study
- 8 - PMID 10695693 - Colorado study
- 9 - PMID 15016491 - Lancet review
- 10 - USPSTF website
- 11 - PMID 25114871 - ETA preg GL
- 12 - PMID 21787128 - ATA preg GL
- 13 - PMID 22869843 - Endocrine Society preg GL
- 14 - PMID 12826640 - Thyroiditis review
- 15 - PMID 21058882 - NHANES subpopulation
- 16 - LabCorp® website
- 17 - CPL® website
- 18 - Quest® diagnostics website
- 19 - Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Walker HK, Hall WD, Hurst JW, editors. Boston: Butterworths; 1990
- 20 - PMID 25266247 - GL for T4 treatment - ATA
- 21 - PMID 21787128 - ATA pregnancy thyroid recs
- 22 - PMID 22869843 - Endocrine Soc preg recs
- 23 - PMID 24783053 - ETA subclinical hypoT4 GL
- 24 - PMID 38877429 - Evaluating the effectiveness of combined T4 and T3 therapy or desiccated thyroid versus T4 monotherapy in hypothyroidism: a systematic review and meta-analysis, BMC Endocr Disord (2024)
- 25 - PMID 22438233 - The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. J Clin Endocrinol Metab, 2012
- 26 - PMID 28056690 - 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease during Pregnancy and the Postpartum, Thyroid, 2016
- 27 - PMID 18815314 - Cellular and Molecular Basis of Deiodinase-Regulated Thyroid Hormone Signaling, Endocrine reviews (2008)
- 28 - PMID 31088853 - Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline, BMJ (2019)
- 29 - PMID 35226058 - Association Between Generic-to-Generic Levothyroxine Switching and Thyrotropin Levels Among US Adults, JAMA Intern Med (2022)
- 30 - Guyton, Arthur C. Guyton And Hall Textbook Of Medical Physiology. Philadelphia, PA : Saunders/Elsevier, 13 ed. (2016)
- 31 - Rhoades, David R. Bell. Medical Physiology : Principles for Clinical Medicine. Philadelphia:Wolters Kluwer Health/Lippincott Williams & Wilkins, 5th ed. (2018)
- 32 - PMID 38124252 - Randomized double-blind placebo-controlled trial on levothyroxine and liothyronine combination therapy in totally thyroidectomized subjects: the LEVOLIO study, Eur J Endocrinol (2024)
