Acronyms
- CEE - Conjugated equine estrogens
- CIMT - Carotid artery intima–media thickness
- COCP - Combined oral contraceptive pill
- EPT - Estrogen plus progestogen therapy
- ET - Estrogen therapy
- FSH - Follicle stimulating hormone
- GnRH - Gonadotropin-releasing hormone
- HPO - Hypothalamic-pituitary-ovarian
- HRT - Hormone replacement therapy
- ICSI - Intracytoplasmic sperm injection
- IUI - Intrauterine insemination
- IVF - In vitro fertilization
- LH - Luteinizing hormone
- MPA - Medroxyprogesterone acetate
- NAMS - North American Menopause Society
- OBS - Observational study
- PCOS - Polycystic ovarian syndrome
- RCT - Randomized controlled trial
- SERM - Selective estrogen receptor modulator
- TLT - Time-lapse embryo culture technology
- TSEC - Tissue-selective estrogen complex
- USPSTF - U.S. Preventive Services Task Force
- VMS - Vasomotor symptoms
- WHI - Women's Health Initiative trials
| FEMALE HORMONES |
|---|
|
Estradiol (E2)
|
|
Estrone (E1)
|
|
Estriol (E3)
|
|
Progesterone
|
|
Follicle-stimulating hormone (FSH)
|
|
Luteinizing hormone (LH)
|
|
Testosterone
|
|
Dehydroepiandrosterone (DHEA)
|
|
Anti-Müllerian hormone
|
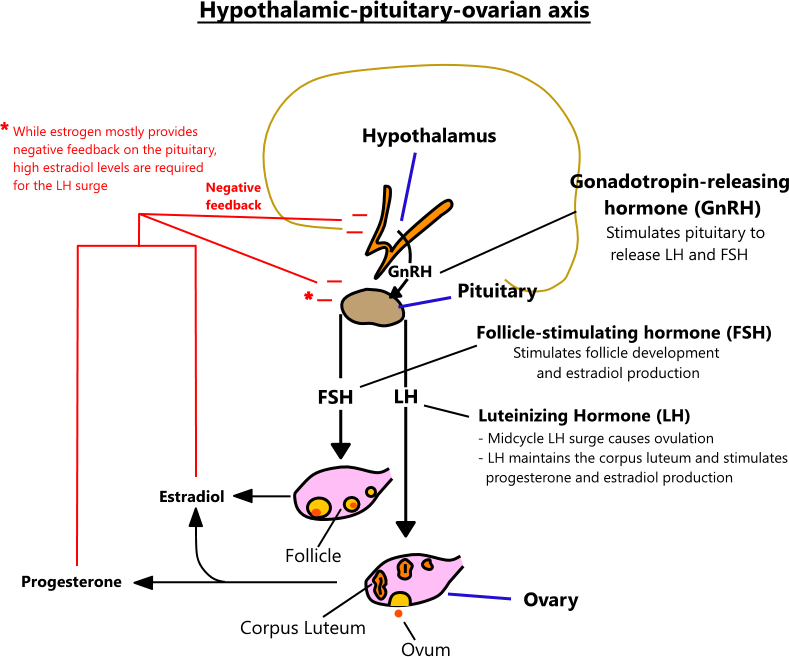
HYPOTHALAMIC-PITUITARY-OVARIAN AXIS
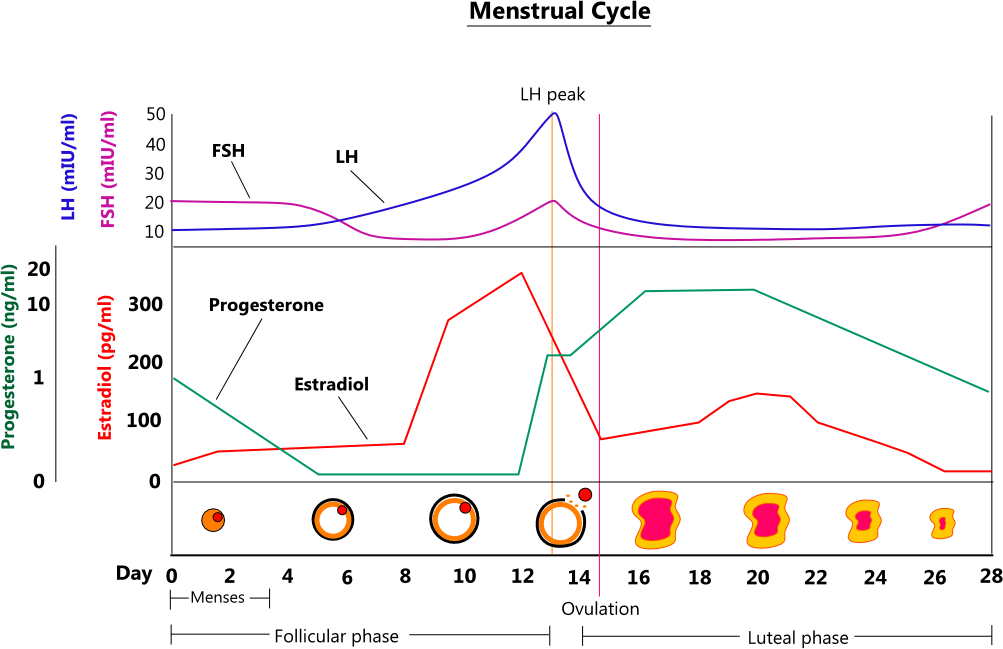
MENSTRUAL CYCLE
- Phases
- Menses (days 0 - 5) - menses occurs during days 0 - 5 of the typical 28-day menstrual cycle. As the corpus luteum regresses, it stops secreting progesterone and estrogen. This causes the endometrium to necrose and slough off.
- Follicular phase (days 1 - 13) - the follicular phase is the first 13 days of a menstrual cycle, where FSH released by the pituitary stimulates the development of a mature follicle. A developing follicle secretes estradiol up to ovulation, at which point it enters the luteal phase.
- Ovulation (days 13 - 14) - ovulation is the release of an egg from a mature follicle, and it typically occurs on days 13 or 14 of a cycle.
- Luteal phase (days 14 - 28) - the final 14 - 28 days of a cycle are the luteal phase. The evacuated follicle becomes the corpus luteum, which releases progesterone and estrogen. Progesterone causes the endometrium to become secretory, preparing it for ovum implantation. Luteinizing hormone stimulates the corpus luteum until it regresses around days 24 - 26, at which point hormone levels plunge, and the endometrium is no longer supported.
POLYCYSTIC OVARY SYNDROME (PCOS)
- PCOS is characterized by polycystic ovaries, irregular menses, and hyperandrogenism. Obesity is present in 50 - 80% of affected individuals, insulin resistance in 35%, and type 2 diabetes in 8 - 10%. It is a common condition, prevalent in 10% of women, and likely underdiagnosed. The pathology is not completely understood but involves abnormalities in ovarian follicular development and steroidogenesis that lead to excessive ovarian androgen production and ovulatory dysfunction. [11,12]
| 2023 PCOS Diagnostic Criteria |
|---|
|
STEP 1 - Assess if the following two criteria are met:
|
|
STEP 2 - Both criteria are met
|
|
STEP 3 - Only one criterion is met
|
| 2023 PCOS Treatment Recommendations |
|---|
|
First-line therapy
|
|
Second-line therapy
|
|
Infertility in PCOS
|
MENOPAUSE
- Overview
- Menopause occurs when ovarian follicles are depleted, and the production of estrogen and progesterone ceases
- Twelve consecutive months without menses is the definition used to mark the beginning of menopause. The median age of the final menstrual period is 52.5 years. Prior to this, women typically experience years of menstrual irregularities. [9]
- Symptoms
- Amenorrhea
- Hot flushes defined as episodic flushing and warmth of the skin often accompanied by sweating
- Vaginal dryness, burning, and irritation
- Sexual symptoms of diminished lubrication and pain
- Urinary urgency, dysuria, and recurrent urinary tract infections
- Irritability and anxiety
- Fatigue and weakness
- Decreased calcification of the bones (osteoporosis)
- Predicting menopause onset
- Menstrual patterns and biomarkers of ovarian reserve can be used to predict the onset of menopause, although neither one is precise
- In general, women who begin menopause at a younger age have a longer transition period and more symptoms than women who start at an older age
- Menstrual patterns
- Early menopause transition - marked by irregularities of ≥ 7 days in the intermenstrual cycle in women with previously normal cycles. This occurs at a median age of 47 years.
- Late menopause transition - amenorrhea for > 60 days, after which 95% of women will go on to have their final menstrual period within the next 4 years. The median age for the late menopause transition is 49 years.
- Menopause - marked by no menses for 12 consecutive months. The median age for the last menstrual period is 52.5 years, and most women go through menopause between the ages of 47 and 54 years. [9]
- Biomarkers of ovarian reserve
- FSH and Anti-Müllerian hormone (AMH) can both be used to predict menopause onset
- FSH levels increase as ovarian reserve decreases. Ideally, FSH levels should be drawn within the first 5 days of menses when they are at their highest. AMH levels fall as ovarian reserve decreases.
- Results from a study that measured AMH and FSH levels in 313 women before following them longitudinally for the onset of menopause are presented in the tables below
| % of women achieving menopause within 5 years based on AMH levels | |||
|---|---|---|---|
| AMH levels | |||
| Age range | Undetectable | 0.09 - 1.9 ng/ml | ≥ 2.0 ng/ml |
| 40 - 44 years (N=192) | 40% | 5% | 0% |
| 45 - 49 years (N=121) | 60% | 23% | 0% |
| % of women achieving menopause within 5 years based on FSH levels | |||
|---|---|---|---|
| FSH levels | |||
| Age range | 0 - 5.39 IU/L | 5.4 - 13 IU/L | > 13 IU/L |
| 40 - 44 years (N=192) | 6% | 6% | 22% |
| 45 - 49 years (N=121) | 31% | 23% | 46% |
- Treatment
- See hormone replacement therapy below and female hormone medications
HORMONE REPLACEMENT THERAPY (HRT)
- Menopausal women often experience a wide range of symptoms that are bothersome and uncomfortable. For years, hormone replacement therapy (HRT) was widely prescribed to treat symptoms and prevent menopause-related conditions (e.g., osteoporosis). However, in 2002, the landmark Women's Health Initiative (WHI) Study was published that found menopausal HRT increased the risk of breast cancer, heart disease, stroke, blood clots, and urinary incontinence. These findings significantly altered the perception of HRT, leading to a steady decline in its use.
- HRT recommendations from the North American Menopause Society (NAMS) and the U.S. Preventive Services Task Force (USPSTF) are provided below along with long-term results from the WHI trial and a review of the ELITE trial, which evaluated the impact of HRT timing on carotid artery intima-media thickness.
| North American Menopause Society 2022 HRT recommendations |
|---|
| HRT indications |
|
| HRT risks and benefits |
Overall
CVD and mortality
Breast cancer
Ovarian cancer
Cognition
|
| Therapy choice |
Women with a uterus
Vasomotor symptoms
Genitourinary symptoms
Urinary tract symptoms
Sexual function
Compounded hormone therapies
|
| Primary ovarian insufficiency (POI) |
|
| Osteoporosis |
|
| Therapy duration and discontiuation |
|
| Special populations |
Women with breast cancer
Breast cancer survivors
Family history of breast cancer
Endometrial cancer
History of ovarian cancer
BRCA-positive women without breast cancer
|
| Nonhormonal therapy |
2023 Recommendations
Suggested dosing
|
- USPSTF 2022 HRT Recommendations
- The USPSTF recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons
- The USPSTF recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy [15]
- HRT patient handouts
- Patient handouts from the NAMS discussing HRT risks, benefits, and treatment options are available at the links below
- Women's health initiative (WHI) trials
- The Women's Health Initiative (WHI) trial, conducted from 1991 to 2005, examined the long-term effects of HRT in postmenopausal women aged 50 to 79. The trial had two arms, one comparing estrogen to placebo in women without a uterus and the other comparing combined estrogen and progesterone to placebo in women with a uterus. Results from each study are summarized in the tables below.
- Estrogen study (Premarin®)
- In the estrogen arm on the WHI, women without a uterus were randomized to conjugated estrogens 0.625 mg/d (N=5310) or placebo (N=5429). The active phase lasted a median of 7.2 years, followed by an additional 6 years of observation, providing a cumulative follow-up of 13 years. Notably, fewer than 4% of participants reported using HRT after the active phase concluded. The primary outcome was the incidence of coronary heart disease and invasive breast cancer. Numerous secondary outcomes were also tracked and reported.
| Premarin® 0.625 daily vs Placebo | |||
|---|---|---|---|
| Outcome | Active phase results (median 7.2 years) | Cumulative results (median 13 years) | Cumulative results (median 18 years) |
| All-cause mortality | HR 1.03 (CI 0.88 - 1.21) NS Case diff = +3 |
HR 0.99 (CI 0.90 - 1.10) NS Case diff = -1 |
HR 0.94 (CI 0.88 - 1.01) NS |
| Coronary heart disease | HR 0.94 (CI 0.78 - 1.14) NS Case diff = -3 |
HR 0.94 (CI 0.82 - 1.09) NS Case diff = -4 |
CHD mortality HR 0.89 (CI 0.75 - 1.05) NS |
| Invasive breast cancer | HR 0.79 (CI 0.61 - 1.02) NS Case diff = -7 |
HR 0.79 (CI 0.65 - 97) S Case diff = -7 |
Breast cancer mortality HR 0.55 (CI 0.33 - 0.92) S |
| Stroke | HR 1.35 (CI 1.07 - 1.70) S Case diff = +11 |
HR 1.15 (CI 0.97 - 1.37) NS Case diff = +5 |
NA |
| Pulmonary embolism | HR 1.35 (CI 0.89 - 2.05) NS Case diff = +4 |
HR 1.15 (CI 0.87 - 1.51) NS Case diff = +2 |
NA |
| Colorectal cancer | HR 1.15 (CI 0.81 - 1.64) NS Case diff = +2 |
HR 1.13 (CI 0.85 - 1.51) NS Case diff = +2 |
Colorectal cancer mortality HR 1.21 (CI 0.79 - 1.84) NS |
| All fractures | HR 0.72 (CI 0.64 - 0.80) S Case diff = -61 |
✝HR 0.91 (CI 0.72 - 1.15) NS Case diff = -2 |
NA |
| Deep vein thrombosis | HR 1.48 (CI 1.06 - 2.07) S Case diff = +7 |
HR 1.05 (CI 0.82 - 1.33) NS Case diff = +1 |
NA |
| Probable dementia (in women ≥ 65 years old only) |
HR 1.47 (CI 0.85 - 2.52) NS Case diff = +15 |
NA | NA |
- Estrogen + progesterone study (Prempro®)
- In the combined estrogen and progesterone arm of the WHI, women with a uterus were randomized to conjugated estrogens 0.625 mg/d + medroxyprogesterone 2.5 mg/d (N=8506 patients) or placebo (N=8102). The active phase lasted a median of 5.6 years, followed by an additional 7 years of observation, providing a cumulative follow-up of 13 years. Notably, fewer than 4% of participants reported using HRT after the active phase concluded. The primary outcome was the incidence of coronary heart disease and invasive breast cancer. Numerous secondary outcomes were also tracked and reported.
| Prempro® 0.625/2.5 mg daily vs Placebo | |||
|---|---|---|---|
| Outcome | Active phase results (median 5.6 years) | Cumulative results (median 13 years) | Cumulative results (median 18 years) |
| All-cause mortality | HR 0.97 (CI 0.81 - 1.16) NS Case diff = -1 |
HR 0.99 (CI 0.91 - 1.08) NS Case diff = -1 |
HR 1.02 (CI 0.96 - 1.08) NS |
| Coronary heart disease | HR 1.18 (CI 0.95 - 1.45) NS Case diff = +6 |
HR 1.09 (CI 0.96 - 1.24) NS Case diff = +3 |
CHD mortality HR 1.05 (CI 0.89 - 1.23) NS |
| Invasive breast cancer | HR 1.24 (CI 1.01 - 1.53) S Case diff = +9 |
HR 1.28 (CI 1.11 - 1.48) S Case diff = +9 |
Breast cancer mortality HR 1.44 (CI 0.97 - 2.15) NS |
| Stroke | HR 1.37 (CI 1.07 - 1.76) S Case diff = +9 |
HR 1.16 (CI 1.00 - 1.35) NS Case diff = +5 |
NA |
| Pulmonary embolism | HR 1.98 (CI 1.36 - 2.87) S Case diff = +9 |
HR 1.26 (CI 1.00 - 1.59) NS Case diff = +4 |
NA |
| Colorectal cancer | HR 0.62 (CI 0.43 - 0.89) S Case diff = -6 |
HR 0.80 (CI 0.63 - 1.01) NS Case diff = -3 |
Colorectal cancer mortality HR 1.01 (CI 0.69 - 1.49) NS |
| Endometrial cancer | HR 0.83 (CI 0.49 - 1.40) NS Case diff = -1 |
HR 0.67 (CI 0.49 - 0.91) S Case diff = -3 |
NA |
| All fractures | HR 0.76 (CI 0.69 - 0.83) S Case diff = -51 |
✝HR 0.81 (CI 0.68 - 0.97) S Case diff = -5 |
NA |
| Deep vein thrombosis | HR 1.87 (CI 1.37 - 2.54) S Case diff = +12 |
HR 1.24 (CI 1.01 - 1.53) S Case diff = +4 |
NA |
| Probable dementia (in women ≥ 65 years old only) |
HR 2.01 (CI 1.19 - 3.42) S Case diff = +23 |
NA | NA |
- ELITE trial
- Observational studies have suggested that the effects of HRT on coronary heart disease depend on the timing of therapy initiation relative to menopause onset. Earlier initiation (less than 10 years from menopause onset) appears to be associated with a beneficial effect, while later initiation (10 years or more since menopause onset) may have no benefit or even a negative effect. To examine the issue, the ELITE trial compared the effects of early vs late HRT initiation on carotid artery intima-media thickness (CIMT), a surrogate measure for atherosclerosis.
RCT
ELITE trial - HRT vs Placebo in Early vs Late Menopause for Carotid Artery Intima-Media Thickness, NEJM (2016) [PubMed abstract]
- The Elite trial enrolled 643 postmenopausal women without a history of coronary artery disease. Participants were stratified according to time since menopause onset, with early postmenopause being less than 6 years since onset and late being 10 years or more.
Main inclusion criteria
- Postmenopausal
- No history of CAD
- No regular menses for at least 6 months or surgically-induced menopause
- Estradiol level < 25 pg/ml
Main exclusion criteria
- Diabetes
- Serum triglycerides > 500 mg/dl
- SBP > 160 | DBP > 110
- History of DVT or PE
- Hormone therapy within 1 month of screening
Baseline characteristics
- Median age: 55 years (early) | 64 years (late)
- Median time since menopause: 3.5 years (early) | 14.3 years (late)
- Median LDL: 136 mg/dl (early) | 132 mg/dl (late)
- Median BP: 116/76 (early) | 118/74 (late)
Randomized treatment groups
- Group 1 (297 patients): Estradiol 1mg once daily. Women with a uterus also received progesterone vaginal gel (45 mg) for 10 days during each 30-day cycle.
- Group 2 (299 patients): Placebo once daily. Women with a uterus also received placebo vaginal gel for 10 days during each 30-day cycle.
- Randomization was stratified based on duration of time since menopause: early postmenopause (< 6 years since menopause), late postmenopause (≥ 10 years since menopause)
Primary outcome: The rate of change in intima–media thickness of the far wall of the right distal common carotid artery, assessed by means of computer image processing of B-mode ultrasonograms that were obtained at two baseline examinations (averaged to obtain the baseline CIMT value) and every 6 months during trial follow-up
Results
| Duration: Median of 5 years | |||
| Outcome | HRT | Placebo | Comparisons |
|---|---|---|---|
| Primary outcome (early postmenopause) | 0.0044 mm/yr | 0.0078 mm/yr | p=0.008 |
| Primary outcome (late postmenopause) | 0.0100 mm/yr | 0.0088 mm/yr | p=0.29 |
|
|||
Findings: Oral estradiol therapy was associated with less progression of subclinical atherosclerosis (measured as CIMT) than was placebo when therapy was initiated within 6 years after menopause but not when it was initiated 10 or more years after menopause. Estradiol had no significant effect on cardiac CT measures of atherosclerosis in either postmenopause stratum.
- Summary
- In the ELITE trial, women receiving HRT early after menopause onset (< 6 years) had a slower rate of CIMT progression compared to those receiving placebo. Conversely, HRT did not affect CIMT progression in women receiving HRT late after menopause onset (≥ 10 years). These findings support observational data suggesting that early initiation of HRT confers greater cardiovascular benefits compared to later initiation. However, the trial is limited by its use of a surrogate endpoint and small sample size.
INFERTILITY
- Infertility is defined as a failure to achieve pregnancy after 12 months of regular unprotected intercourse. In the U.S., about 8.8% of women aged 15 - 49 years are affected.
- The recommendations below provide a straightforward approach to initiating an infertility evaluation [10]
| Steps to evaluating infertility |
|---|
Step 1 - Determine if patient is ovulating
|
Step 2 - Order testing based on ovulation status
|
|
Step 3 - Considerations based on results
|
| Normal semen parameters | |
|---|---|
| Measure | Normal value |
| Sperm concentration |
|
| Motility |
|
| Total motile sperm count (TMC) |
|
| Sperm morphology |
|
| White blood cell count |
|
| Infertility drugs |
|---|
|
Clomiphene (Clomid®)
|
|
Letrozole (Femara®)
|
|
Gonadotropins (Gonal-f®, Menopur®)
|
STUDIES | INFERTILITY
- RCTFSH vs Clomiphene ± Intrauterine Insemination (IUI) in Unexplained Infertility, Lancet (2018) [PubMed abstract]
- Design: Randomized, controlled trial (N=666, length = up to 6 cycles) in women who had used clomiphene for 6 cycles and not conceived
- Treatment: FSH + HCG vs Clomiphene 50 - 150 mg/day. Both groups were also randomly assigned to IUI or regular intercourse.
- Primary outcome: Conception leading to livebirth within 8 months after randomisation, defined as any baby born alive with a gestational age beyond 24 weeks
- Results:
- Livebirth: FSH/HCG + IUI - 54%, FSH/HCG + intercourse - 48%, Clomiphene + IUI - 44%, Clomiphene + intercourse - 39% (FSH/HCG vs Clomiphene p=0.0124 | IUI vs intercourse p=0.12)
- Findings: In women with normogonadotropic anovulation and clomiphene citrate failure, a switch of treatment to gonadotropins increased the chance of livebirth over treatment with clomiphene citrate; there was no evidence that addition of intrauterine insemination does so.
- RCTClomiphene or Letrozole + Intrauterine Insemination (IUI) vs Expectant Management for Unexplained Infertility, Lancet (2018) [PubMed abstract]
- Design: Randomized, controlled trial (N=201, length = up to 3 cycles) in women with unexplained infertility
- Treatment: Clomiphene 50 - 150 mg/day or letrozole 2.5 - 7.5 mg/day for 5 days + IUI vs Expectant management (no intervention)
- Primary outcome: Cumulative live birth rate in the intention-to-treat population
- Results:
- Live birth: Ovarian stimulation + IUI - 31%, Expectant management - 9% (p=0.0003)
- Clinical pregnancy: Ovarian stimulation + IUI - 37%, Expectant management - 11% (p<0.0001)
- Multiple pregnancies (out of live births): Ovarian stimulation + IUI - 6%, Expectant management - 0%
- Findings: IUI with ovarian stimulation is a safe and effective treatment for women with unexplained infertility and an unfavourable prognosis for natural conception
- RCTLetrozole vs Clomid vs Gonadotropin (Menopur) for Unexplained Infertility, NEJM (2015) [PubMed abstract]
- Design: Randomized, controlled trial (N=900, length = up to 4 cycles) in women with unexplained infertility
- Treatment: Letrozole 2.5 - 7.5 mg/day for 5 days vs Clomiphene 100 mg/day for 5 days vs Gonadotropin (Menopur) daily until hCG | All groups received 10,000 IU of hCG upon follicle development | All groups received intrauterine insemination
- Primary outcome: Rate of multiple gestations among women with clinical pregnancies
- Results:
- Multiple gestations (among clinical pregnancies): Gonadotropin - 32%, Clomiphene - 9%, Letrozole - 13%
- Clinical pregnancy: Gonadotropin - 36%, Clomiphene - 28%, Letrozole - 22%
- Live births (among all women): Gonadotropin - 32%, Clomiphene - 23%, Letrozole - 19%
- Findings: In women with unexplained infertility, ovarian stimulation with letrozole resulted in a significantly lower frequency of multiple gestation but also a lower frequency of live birth, as compared with gonadotropin but not as compared with clomiphene.
- RCTClomiphene vs FSH vs Immediate IVF for Unexplained Infertility, Fertil Steril (2014) [PubMed abstract]
- Design: Randomized, controlled trial (N=154, length = up to 2 cycles) in women with > 6 months of unexplained infertility
- Treatment: Clomiphene 100 mg/day for 5 days vs FSH 300 IU/day for 3 days vs Immediate IVF | Clomiphene group received 10,000 IU of hCG if necessary | FSH groups received 10,000 IU of hCG upon follicle development | Clomiphene/FSH groups received intrauterine insemination
- Primary outcome: Proportion with a clinically recognized pregnancy, number of treatment cycles, and time to conception after two treatment cycles and at the end of treatment.
- Results:
- Clinical pregnancy: Clomiphene - 22%, FSH - 17%, IVF - 49%
- Live births: Clomiphene - 16%, FSH - 14%, IVF - 31%
- Findings: A randomized controlled trial in older women with unexplained infertility to compare treatment initiated with two cycles of controlled ovarian hyperstimulation/IUI versus immediate IVF demonstrated superior pregnancy rates with fewer treatment cycles in the immediate IVF group.
- OBSBirth Outcomes in Medically Assisted Reproduction vs Natural Reproduction, Lancet (2019) [PubMed abstract]
- Design: Registry cohort study (N=65,723, length = 5 years) in households with at least one child aged 0 - 14 years
- Exposure: Medically assisted reproduction (ovulation induction, artificial insemination, IVF, and/or ICSI with fresh or frozen embryo transfers) vs Natural reproduction
- Primary outcome: Birthweight, gestational age, risk of low birthweight, and risk of preterm birth
- Results:
- Birthweight (grams): Medically assisted - 3286, Natural - 3551 (p<0.0001)
- Low birthweight: Medically assisted - 13%, Natural - 3% (p<0.0001)
- Gestational age (days): Medically assisted - 271, Natural - 278 (p<0.0001)
- Preterm birth: Medically assisted - 15%, Natural - 5% (p<0.0001)
- Findings: Children conceived by medically assisted reproduction face an elevated risk of adverse birth outcomes. However, our results indicate that this increased risk is largely attributable to factors other than the medically assisted reproduction treatment itself
- OBSAssociation Between Fertility Treatment and Cancer Risk in Children, JAMA (2019) [PubMed abstract]
- Design: Registry cohort study (N=1,085,172 | length = 11.3 years) in Danish children born between 1996 and 2012
- Exposure: Maternal fertility treatment including the use of fertility drugs (clomiphene, gonadotropins, gonadotropin-releasing hormone analogs, human chorionic gonadotropin, progesterone, and estrogen) and assisted reproductive technology (in vitro fertilization, intracytoplasmic sperm injection, and frozen embryo transfer) vs None
- Primary outcome: Hazard ratios and incidence rate differences for childhood cancer
- Results:
- Only frozen embryo transfer showed a significantly higher incidence of cancer compared to no treatment. (Incidence rate difference, 26.9 cases/100000 children, 95%CI[2.8 - 51.0])
- Findings: Among children born in Denmark, the use of frozen embryo transfer, compared with children born to fertile women, was associated with a small but statistically significant increased risk of childhood cancer; this association was not found for the use of other types of fertility treatment examined.
STUDIES | INFERTILITY IN PCOS
- RCTLetrozole vs Clomiphene for Infertility in PCOS, NEJM (2014) [PubMed abstract]
- Design: Randomized controlled trial (N=750, length = up to 5 cycles) in infertile women with PCOS
- Treatment: Letrozole 2.5 - 7.5 mg/day for 5 days vs Clomiphene 50 - 150 mg/day for 5 days | Both drugs were started on cycle day 3
- Primary outcome: Live births during the treatment period
- Results:
- Live births: Letrozole - 27.5%, Clomiphene - 19.1% (p=0.007)
- Twin live births: Letrozole - 3.4%, Clomiphene - 7.4% (p=0.32)
- Findings: As compared with clomiphene, letrozole was associated with higher live-birth and ovulation rates among infertile women with the polycystic ovary syndrome.
- RCTClomiphene vs Metformin vs Clomiphene + Metformin for Infertility in PCOS, NEJM (2007) [PubMed abstract]
- Design: Randomized controlled trial (N=626, length = up to 6 months) in infertile women with PCOS
- Exposure: Metformin 1000 mg twice daily vs Clomiphene 50 - 150 mg/day for 5 days beginning on day 3 of menses vs Both treatments
- Primary outcome: Rate of live births
- Results:
- Live births: Clomiphene - 23%, Metformin 7%, Clomiphene + Metformin - 27% (p<0.001 for metformin vs both clomiphene and combination therapy | p=0.31 for clomiphene vs combination therapy)
- Multiple pregnancies (among all pregnancies): Clomiphene - 6%, Metformin 0%, Clomiphene + Metformin - 3%
- Findings: Clomiphene is superior to metformin in achieving live birth in infertile women with the polycystic ovary syndrome, although multiple birth is a complication.
STUDIES | IVF
ICSI studies
RCT
Intracytoplasmic Sperm Injection vs Conventional IVF in Non-severe Male Factor Infertility, Lancet (2024) [PubMed abstract]
- Design: Randomized controlled trial (N=2387, length = 1 transfer) in couples with infertility with non-severe male factor without a history of poor fertilization
- Treatment: Intracytoplasmic sperm injection vs Conventional IVF
- Primary outcome: Livebirth after the first embryo transfer
- Results:
- Live births: Intracytoplasmic injection - 33.8%, Conventional IVF - 36.6% (p=0.16)
- Findings: In couples with infertility with non-severe male factor, ICSI did not improve live birth rate compared with conventional IVF. Given that ICSI is an invasive procedure associated with additional costs and potential increased risks to offspring health, routine use is not recommended in this population.
RCT
Intracytoplasmic Sperm Injection vs Conventional IVF When Sperm Count and Mobility are Normal, Lancet (2021) [PubMed abstract]
- Design: Randomized controlled trial (N=1064, length = 1 transfer) in couples who had undergone ≤ 2 previous conventional IVF or intracytoplasmic sperm injection attempts where the male had normal sperm count and mobility
- Treatment: Intracytoplasmic sperm injection vs Conventional IVF
- Primary outcome: Livebirth after the first embryo transfer from the initiated cycle
- Results:
- Live births: Intracytoplasmic injection - 35%, Conventional IVF - 31% (p=0.27)
- Findings: In couples with infertility in whom the male partner has a normal total sperm count and motility, intracytoplasmic sperm injection did not improve the livebirth rate compared with conventional IVF. Our results challenge the value of the routine use of intracytoplasmic sperm injection in assisted reproduction techniques for this population.
RCT
Physiological, hyaluronan-selected ICSI vs Standard ICSI for infertility treatment, Lancet (2019)
[PubMed abstract]
- Design: Randomized controlled trial (N=2772, length = 1 transfer of 1 - 3 fresh embryos) in women undergoing IVF with embryos formed from intracytoplasmic sperm injection (ICSI)
- Treatment: Hyaluronan-based sperm selection for ICSI (so-called physiological ICSI [PICSI]) vs standard ICSI
- Primary outcome: Full-term (≥37 weeks’ gestational age) livebirth
- Results:
- Live births: Physiological ICSI (PICSI) - 27.4%, Standard ICSI - 25.2% (p=0.18)
- Findings: Compared with ICSI, PICSI does not significantly improve term livebirth rates. The wider use of PICSI, therefore, is not recommended at present.
Endometrial procedures
RCT
Timing by Endometrial Receptivity Testing vs Standard Timing in IVF, JAMA (2022) [PubMed abstract]
- Design: Double-blind, randomized controlled trial (N=767 | length = 1 transfer) in women undergoing IVF with a euploid blastocyst(s)
- Treatment: Receptivity-timed frozen embryo transfer with adjusted duration of progesterone exposure prior to transfer vs Standard timing
- Primary outcome: Live birth
- Results:
- Live births: Receptivity timing - 58.5%, Standard timing - 61.9% (p=0.38)
- Findings: Among patients for whom in vitro fertilization yielded a euploid blastocyst, the use of receptivity testing to guide the timing of frozen embryo transfer, compared with standard timing for transfer, did not significantly improve the rate of live birth. The findings do not support routine use of receptivity testing to guide the timing of embryo transfer during in vitro fertilization.
RCT
Endometrial Scratching vs None Before IVF, NEJM (2019)
[PubMed abstract]
- Design: Randomized controlled trial (N=1364, length = 1 transfer) in women planning IVF with their own oocytes
- Treatment: Endometrial scratching vs No endometrial scratching
- Primary outcome: Live birth
- Results:
- Live births: Endometrial scratching - 26.1%, No endometrial scratching - 26.1%
- Findings: Endometrial scratching did not result in a higher rate of live birth than no intervention among women undergoing IVF
RCT
Endometrial Preparation vs None Before IVF, Lancet (2024)
[PubMed abstract]
- Design: Randomized controlled trial (N=1428, length = 1 transfer) in ovulatory women undergoing frozen embryo transfer
- Treatment: Modified natural cycle (hCG to trigger ovulation) vs Artificial cycle (oral estradiol and vaginal progesterone to mimic a cycle) vs Natural cycle (no hormones)
- Primary outcome: Livebirth after one frozen embryo transfer
- Results:
- Primary outcome: Modified natural cycle - 33%, Artificial cycle - 34%, Natural cycle - 37% (all comparisons nonsignificant)
- Findings: Although the livebirth rate was similar after natural, modified natural, and artificial cycle endometrial preparation strategies in ovulatory women undergoing FET IVF, no definitive conclusions can be made regarding the comparative safety of the three approaches.
Frozen vs fresh embryos
RCT
Transfer of Frozen vs Fresh Embryos in IVF, NEJM (2018)
[PubMed abstract]
- Design: Randomized controlled trial (N=2157, length = 1 transfer) in ovulatory women with infertility due to tubal factors, male factors, or both
- Treatment: Transfer of fresh embryos vs frozen embryos
- Primary outcome: Live birth (≥ 28 weeks gestation) after the first transfer
- Results:
- Live births: Frozen - 49%, Fresh - 50% (p=0.50)
- Findings: The live-birth rate did not differ significantly between fresh-embryo transfer and frozen-embryo transfer among ovulatory women with infertility, but frozen-embryo transfer resulted in a lower risk of the ovarian hyperstimulation syndrome.
RCT
Transfer of Frozen vs Fresh Embryos in IVF in Women Without PCOS, NEJM (2018)
[PubMed abstract]
- Design: Randomized controlled trial (N=782, length = 1 transfer) in infertile women without PCOS
- Treatment: Transfer of fresh embryos vs frozen embryos
- Primary outcome: Pregnancy after first transfer defined as pregnancy with a detectable heart rate after 12 weeks of gestation
- Results:
- Live births: Frozen - 36%, Fresh - 35% (p=0.65)
- Findings: Among infertile women without the polycystic ovary syndrome who were undergoing IVF, the transfer of frozen embryos did not result in significantly higher rates of ongoing pregnancy or live birth than the transfer of fresh embryos.
RCT
Transfer of Frozen vs Fresh Single Blastocyst in IVF, Lancet (2019)
[PubMed abstract]
- Design: Randomized controlled trial (N=1650, length = 1 transfer) in women with regular menstrual cycles undergoing their first cycle of IVF
- Treatment: Single fresh blastocyst transfer vs Single frozen blastocyst transfer
- Primary outcome: Singleton livebirth rate
- Results:
- Live births: Frozen - 50%, Fresh - 40% (p<0.0001)
- Findings: Frozen single blastocyst transfer resulted in a higher singleton livebirth rate than did fresh single blastocyst transfer in ovulatory women with good prognosis. The increased risk of pre-eclampsia after frozen blastocyst transfer warrants further studies.
RCT
Freeze-all Embryos vs Fresh Transfer of an Embryo in Women with Fertility Issues, BMJ (2020)
[PubMed abstract]
- Design: Randomized controlled trial (N=460, length = 1 transfer) in women with regular menstrual cycles undergoing IVF (cycles 1, 2, or 3) for male, tubal, uterine, or unexplained infertility
- Treatment: Freeze-all embryos and transfer one after one completed menstrual cycle vs Transfer fresh embryo on day 5 of culture
- Primary outcome: Ongoing pregnancy rate defined as a detectable fetal heart beat after eight weeks of gestation
- Results:
- Primary outcome: Freeze-all - 27.8%, Fresh - 29.6% (p=0.76)
- No significant differences between groups were observed for positive human chorionic gonadotropin rate or pregnancy loss, and none of the women had severe ovarian hyperstimulation syndrome
- Findings: In women with regular menstrual cycles, a freeze-all strategy with gonadotropin releasing hormone agonist triggering for final oocyte maturation did not result in higher ongoing pregnancy and live birth rates than a fresh transfer strategy. The findings warrant caution in the indiscriminate application of a freeze-all strategy when no apparent risk of ovarian hyperstimulation syndrome is present.
Other IVF studies
RCT
TLT vs EEVA-TLT vs No TLT for Embryo Selection in IVF, Lancet (2023)
[PubMed abstract]
- Definitions: Time-lapse embryo culture technology (TLT) is an embryo selection method that allows the continuous, dynamic assessment of embryo morphological changes without the need to remove embryos from the incubator. Early Embryo Viability Assessment (EEVA) is a type of TLT that uses an algorithm for assessing embryos in the first 3 days of growth.
- Design: Randomized controlled trial (N=1731, length = 12 months) in couples undergoing IVF or intracytoplasmic sperm injection
- Treatment: TLT vs EEVA-TLT vs No TLT
- Primary outcome: The co-primary endpoints were the cumulative ongoing pregnancy rate within 12 months in all women and the ongoing pregnancy rate after fresh single embryo transfer in a good prognosis population
- Results:
- 12-month cumulative pregnancy rate: TLT - 50.9%, EEVA-TLT - 50.8%, No TLT - 49.4% (p=0.85)
- Good prognosis population: TLT - 36.8%, EEVA-TLT - 38.2%, No TLT - 37.8% (p=0.90)
- Findings: Neither time-lapse-based embryo selection using the EEVA test nor uninterrupted culture conditions in a time-lapse incubator improved clinical outcomes compared with routine methods. Widespread application of time-lapse monitoring for fertility treatments with the promise of improved results should be questioned.
RCT
TLT for Culture and Selection vs None in IVF, Lancet (2024)
[PubMed abstract]
- Definitions: Time-lapse embryo culture technology (TLT) is an embryo selection method that allows the continuous, dynamic assessment of embryo morphological changes without the need to remove embryos from the incubator
- Design: Randomized controlled trial (N=1575, length = 1 round) in couples receiving their first, second, or third IVF or ICSI treatment
- Treatment: TLT for culture and embryo selection (TLT-CS group) vs TLT for culture alone (TLT-C group) vs No TLT
- Primary outcome: Live birth
- Results:
- Primary outcome: TLT-CS - 33.7%, TLT-C - 36.6%, No TLT - 33% (all comparisons nonsignificant)
- Findings: In women undergoing IVF or ICSI treatment, the use of time-lapse imaging systems for embryo culture and selection does not significantly increase the odds of live birth compared with standard care without time-lapse imaging.
RCT
Preimplantation Genetic Testing for Aneuploidy vs None, NEJM (2021) [PubMed abstract]
- Design: Randomized controlled trial (N=1212 | length = 1 year) in women aged 20 - 37 years with subfertility and the availability of three or more good-quality blastocysts (defined as having good morphologic criteria)
- Treatment: Preimplantation genetic testing for aneuploidy (PGT-A) vs None. Only single frozen-embryo transfers were performed each time. Women could receive up to 3 transfers within a year.
- Primary outcome: Cumulative livebirth rate that resulted from up to three embryo transfers performed within 1 year after randomization
- Results:
- Primary outcome: PGT-A - 77.2%, None - 81.8% (HR 0.94, 95%CI [0.89 - 1.00])
- Cumulative pregnancy loss: PGT-A - 8.7%, None - 12.6% (HR 0.69, 95%CI [0.49 - 0.98])
- Good birth outcome: PGT-A - 62.4%, None - 63.5% (HR 0.98, 95%CI [0.90 - 1.07])
- Good birth outcome defined as a live birth at 37 weeks or more of gestation, with a birth weight between 2500 and 4000 g and without a major congenital anomaly.
- Findings: Among women with three or more good-quality blastocysts, conventional IVF resulted in a cumulative live-birth rate that was noninferior to the rate with PGT-A.
OBS
Association of IVF With Childhood Cancer in the United States, JAMA Pediatrics (2019) [PubMed abstract]
- Design: Retrospective cohort study (N=2,542,533, length = 10 years) in children born in the United States
- Exposure: Conceived via IVF vs Not conceived via IVF
- Primary outcome: Cancer diagnosed in the first decade of life
- Results:
- Cancer rate (per 1,000,000 person-years): IVF - 252, Non-IVF - 193 (HR 1.17; 95%CI [1.00-1.36])
- Findings: This study found a small association of IVF with overall cancers of early childhood, but it did observe an increased rate of embryonal cancers, particularly hepatic tumors, that could not be attributed to IVF rather than to underlying infertility. Continued follow-up for cancer occurrence among children conceived via IVF is warranted.
RCT
Prednisone vs Placebo for Live Birth in Recurrent IVF Failure, JAMA (2023) [PubMed abstract]
- Design: Randomized, placebo-controlled trial (N=715 | length = 1 round) in women who had a history of 2 or more unsuccessful embryo transfer cycles, were younger than 38 years when oocytes were retrieved, and were planning to undergo frozen-thawed embryo transfer with the availability of good-quality embryos
- Treatment: Prednisone 10 mg once daily vs Placebo from the day at which they started endometrial preparation for frozen-thawed embryo transfer through early pregnancy
- Primary outcome: Live birth, defined as the delivery of any number of neonates born at 28 or more weeks' gestation with signs of life
- Results:
- Primary outcome: Prednisone - 37.8%, Placebo - 38.8% (p=0.78)
- Findings: Among patients with recurrent implantation failure, treatment with prednisone did not improve live birth rate compared with placebo. Data suggested that the use of prednisone may increase the risk of preterm delivery and biochemical pregnancy loss. Our results challenge the value of prednisone use in clinical practice for the treatment of recurrent implantation failure.
STUDIES | OTHER
- OBSAssociation Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age, JAMA (2017) [PubMed abstract]
- Design: Prospective cohort study (N=750, length = up to 12 cycles) in women aged 30 to 44 years without a history of infertility who had been trying to conceive for ≤ 3 months
- Measured variables: Early-follicular-phase serum level of Antimüllerian hormone (AMH), follicle-stimulating hormone (FSH), and inhibin B and urinary level of FSH
- Primary outcome: Cumulative probability of conception by 6 and 12 cycles of attempt and relative fecundability (probability of conception in a given menstrual cycle). Conception was defined as a positive pregnancy test result.
- Results:
- Conception during study (% of women) by Antimüllerian hormone level
- < 0.7 ng/ml (N=84): 63%
- 0.7 - 8.4 ng/ml (N=579): 66%
- ≥ 8.5 ng/ml (N=74): 59%
- Findings: Among women aged 30 to 44 years without a history of infertility who had been trying to conceive for 3 months or less, biomarkers indicating diminished ovarian reserve compared with normal ovarian reserve were not associated with reduced fertility. These findings do not support the use of urinary or blood follicle-stimulating hormone tests or antimüllerian hormone levels to assess natural fertility for women with these characteristics.
| SERM ACTIVITY TABLE | ||||||
|---|---|---|---|---|---|---|
| SERM | Product | Endometrium | Vagina | Breast | Bone formation |
Hypothalamus |
| Bazedoxifene | Duavee® | Neutral to Antagonist |
No data | Neutral to Antagonist |
Agonist | ? |
| Clomiphene | Clomid® | Antagonist | ? | ? | Agonist | Antagonist |
| Ospemifene | Osphena® | Neutral to Partial agonist |
Agonist | Antagonist | Agonist | ? |
| Raloxifene | Evista® | Neutral to Partial agonist |
Neutral | Antagonist | Agonist | ? |
| Tamoxifen | Nolvadex® | Neutral to Agonist |
Agonist | Antagonist | Agonist | ? |
BIBLIOGRAPHY
- 1 - LabCorp® website
- 2 - CPL® website
- 3 - PMID 17125587 NEJM Menopause review
- 4 - Clomiphene PI
- 5 - PMID 17287476 - clomiphene and metformin in PCOS
- 6 - PMID 26398071 - clomiphene vs letrozole vs gonadotropin
- 7 - PMID 25006718 - letrozole vs clomiphene in PCOS
- 8 - PMID 28610678 - Anti-Müllerian hormone, follicle stimulating hormone, antral follicle count, and risk of menopause within 5 years, Maturitas (2017)
- 9 - PMID 31329213 - Diagnosing the Onset of Menopause, JAMA (2019)
- 10 - PMID 34228062 - Diagnosis and Management of Infertility, JAMA (2021)
- 11 - International Evidence-based Guideline for the assessment and management of polycystic ovary syndrome 2023
- 12 - PMID 27406348 - CLINICAL PRACTICE. Polycystic Ovary Syndrome, NEJM (2016)
- 13 - PMID 30604525 - The efficacy and use of finasteride in women: a systematic review, Int J Dermatol (2019)
- 14 - PMID 35797481 - The 2022 hormone therapy position statement of The North American Menopause Society, Menopause (2022)
- 15 - USPSTF website
- 16 - PMID 24084921 - Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials, JAMA (2013)
- 17 - PMID 28898378 - Menopausal Hormone Therapy and Long-term All-Cause and Cause-Specific Mortality, JAMA (2017)
